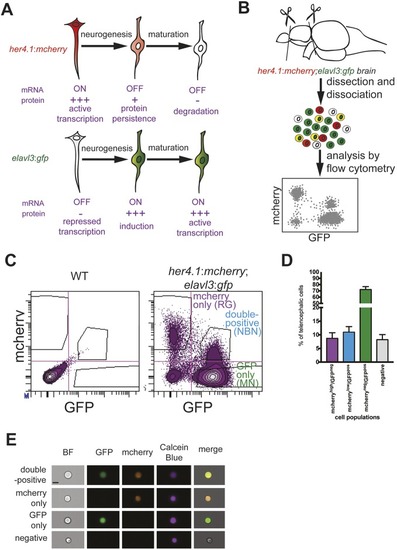
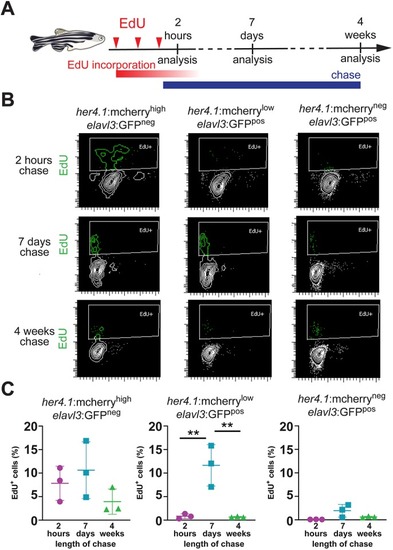
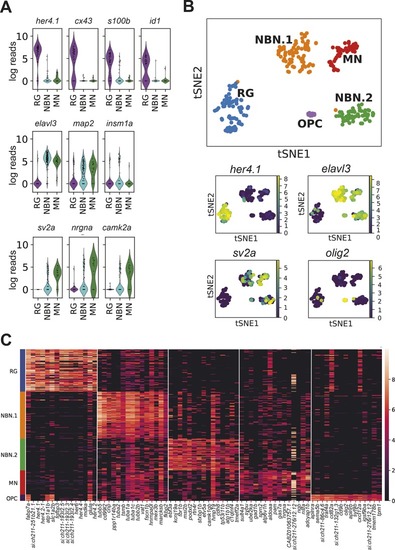
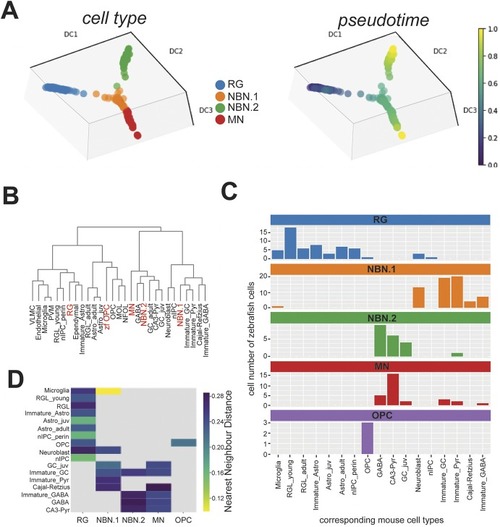
- Title
-
Single cell sequencing of radial glia progeny reveals diversity of newborn neurons in the adult zebrafish brain
- Authors
- Lange, C., Rost, F., Machate, A., Reinhardt, S., Lesche, M., Weber, A., Kuscha, V., Dahl, A., Rulands, S., Brand, M.
- Source
- Full text @ Development
|
|
|
|
|
|
|
|
|
|
|
|