- Title
-
Bi-allelic variants in RNF170 are associated with hereditary spastic paraplegia
- Authors
- Wagner, M., Osborn, D.P.S., Gehweiler, I., Nagel, M., Ulmer, U., Bakhtiari, S., Amouri, R., Boostani, R., Hentati, F., Hockley, M.M., Hölbling, B., Schwarzmayr, T., Karimiani, E.G., Kernstock, C., Maroofian, R., Müller-Felber, W., Ozkan, E., Padilla-Lopez, S., Reich, S., Reichbauer, J., Darvish, H., Shahmohammadibeni, N., Tafakhori, A., Vill, K., Zuchner, S., Kruer, M.C., Winkelmann, J., Jamshidi, Y., Schüle, R.
- Source
- Full text @ Nat. Commun.
|
|
|
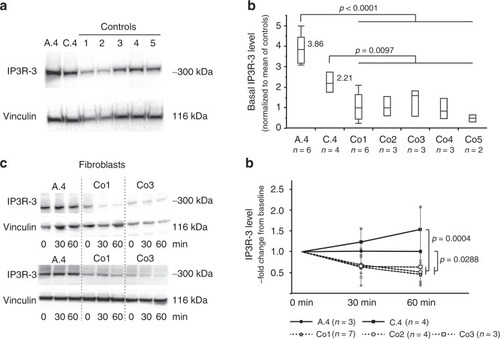
Loss of RNF170 results in decreased degradation of IP3R-3 in patient fibroblasts. |
|
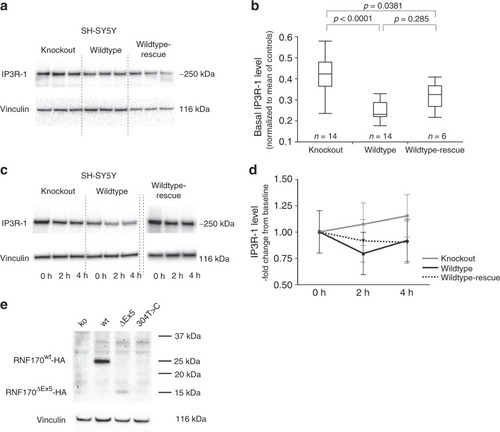
Effect of RNF170 mutations on IP3R-1 degradation and abundance in neuronal cells. |
|
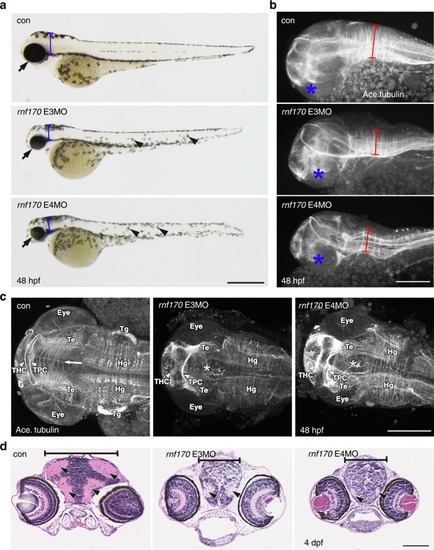
MO knockdown of |
|
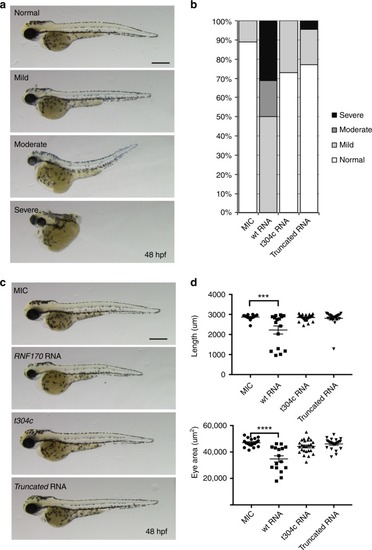
Overexpression of mutant |
|
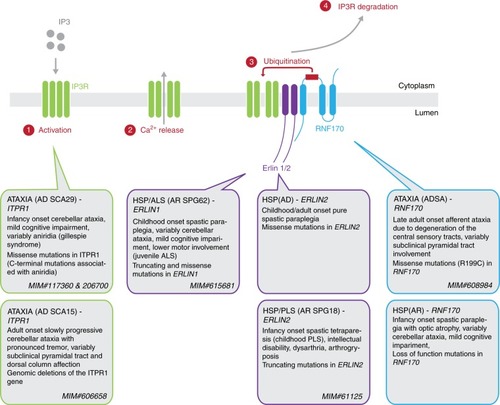
RNF170-dependent degradation of activated IP3R and genetic disorders affecting this pathway. Upon activation of the IP3R with IP3, calcium is released from the ER into the cytoplasm. This triggers the association of IP3R with the ERLIN1/2 complex leading to the ubiquitination of IP3R by the E3 ubiquitin ligase RNF170, resulting in the proteasomal degradation of IP3R. Mutations in all genes encoding components of this pathway are known to cause hereditary neurologic disorders, especially spastic paraplegia and spinocerebellar ataxia |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|