- Title
-
Rewiring the Regenerated Zebrafish Retina: Reemergence of Bipolar Neurons and Cone-Bipolar Circuitry Following an Inner Retinal Lesion
- Authors
- McGinn, T.E., Galicia, C.A., Leoni, D.C., Partington, N., Mitchell, D.M., Stenkamp, D.L.
- Source
- Full text @ Front Cell Dev Biol
|
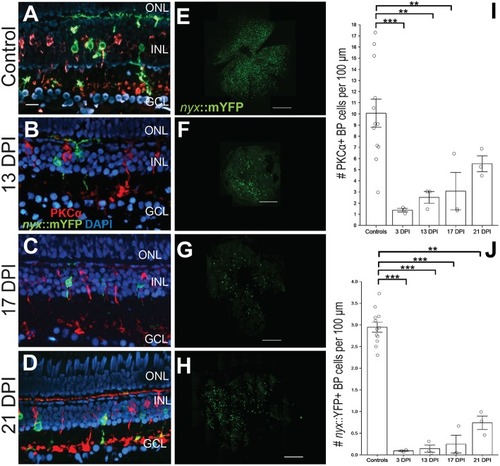
Emergence of identifiable retinal bipolar (BP) neurons following a chemical lesion selective to the inner retina. |
|
Comparative birthdating of regenerated PKCα+ bipolar (BP) neurons and HuC/D+ amacrine and ganglion cells following a chemical lesion selective to the inner retina. |
|
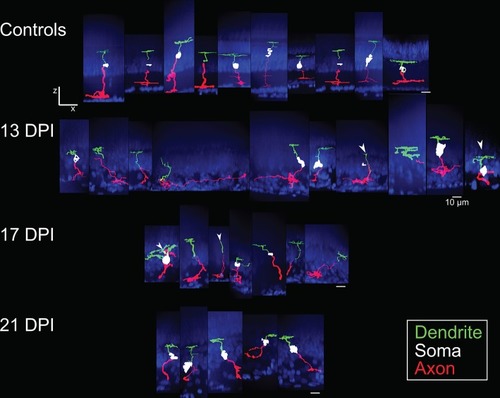
Galleries of traced |
|
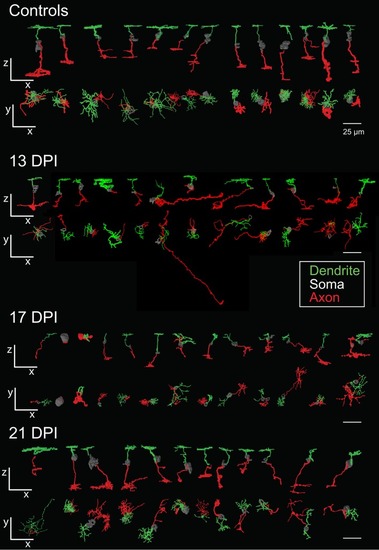
Galleries of surface rendered |
|
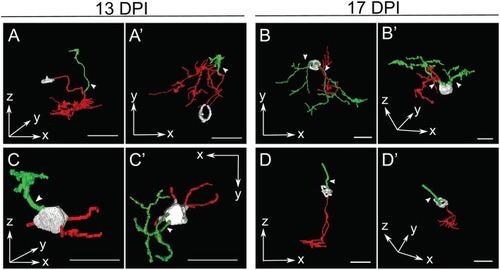
Selected retinal bipolar (BP) neurons that demonstrate the unusual range of morphologies observed at 13 and 17 days post-injury (DPI). Neurons were traced in Simple Neurite Tracer and then rendered in ImageJ's 3D viewer. |
|
Reestablishment of |