- Title
-
IGF-Binding Proteins: Why Do They Exist and Why Are There So Many?
- Authors
- Allard, J.B., and Duan, C.
- Source
- Full text @ Front Endocrinol (Lausanne)
|
|
|
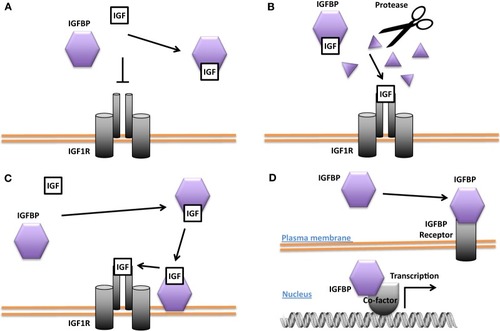
Different modes of Insulin-like growth factor-binding protein (IGFBP) actions. |
|
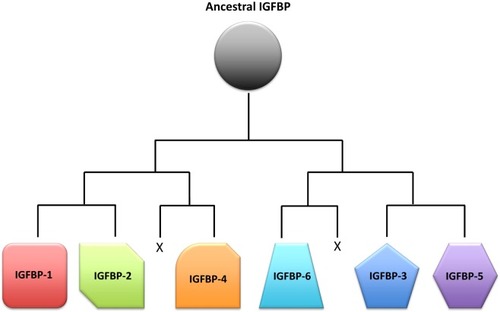
Schematic representation of a proposed scenario of the insulin-like growth factor-binding protein (IGFBP) family evolution. A single ancestral IGFBP gene was duplicated in an early chordate. This duplication was followed by two successive rounds of chromosomal duplications or tetraploidization events in early vertebrates. Of the eight IGFBPs that resulted from this process, two were subsequently lost, leaving six types of IGFBPs that are seen in modern vertebrates. |
|
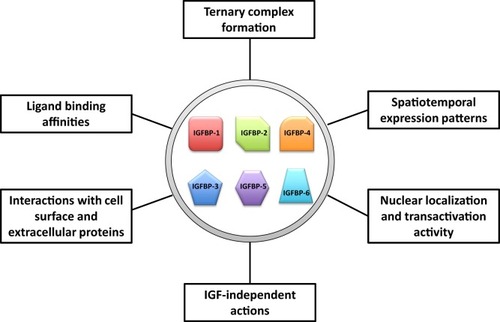
Major attributes of insulin-like growth factor-binding proteins (IGFBPs) that may help to give rise to the increased flexibility and versatility in their abilities to regulate insulin-like growth factor (IGF) actions. |