- Title
-
Transmission Disrupted: Modeling Auditory Synaptopathy in Zebrafish
- Authors
- Kindt, K.S., Sheets, L.
- Source
- Full text @ Front Cell Dev Biol
|
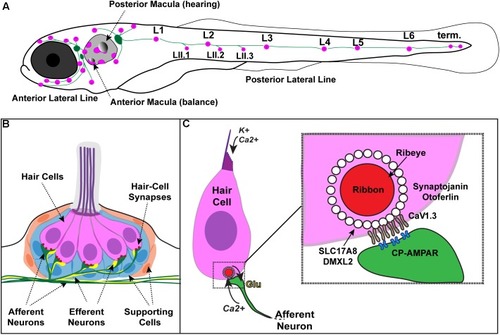
Zebrafish hair cells and ribbons synapses. |
|
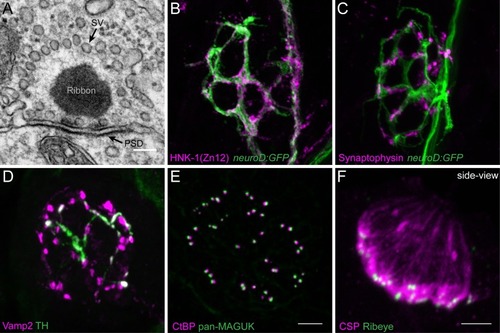
Morphological examination of hair-cell synapses in zebrafish. |
|
Functional analysis of hair-cell synapses in the zebrafish lateral line. |