- Title
-
Hspb7 is a Cardioprotective Chaperone Facilitating Sarcomeric Proteostasis
- Authors
- Mercer, E.J., Lin, Y.F., Cohen-Gould, L., Evans, T.
- Source
- Full text @ Dev. Biol.
|
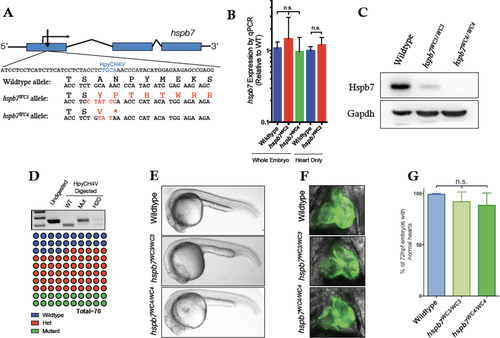
Generation of mutant hspb7 zebrafish lines. (A) Structure and partial sequence of the hspb7 gene, showing TALEN targeting proximal to the start codon and resultant mutant alleles WC3 and WC4. (B) qPCR data showing that hspb7WC3 and hspb7WC4 transcripts are expressed at levels comparable to wildtype hspb7 in 5 dpf zebrafish embryos. (C) Western blot of protein lysates from adult zebrafish hearts showing loss of Hspb7 protein in animals homozygous for either of the hspb7 mutant alleles. (D) RFLP analysis showing loss of the HpyCH4V cut site in PCR product from hspb7WC3/WC3 embryos. Quantification of embryonic genotypes demonstrates that hspb7WC3/WC3 alleles are transmitted at Mendelian ratios. (E) Representative brightfield images of wildtype or homozygous hspb7 mutant 24 hpf embryos. (F) Representative 72 hpf hearts from wildtype or hspb7 mutant lines crossed onto a tg(myl7:gfp) background. (G) Quantification of normal hearts at 72 hpf in wildtype or homozygous hspb7 mutant animals (n = 200). PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
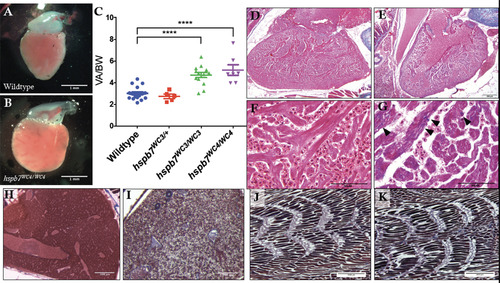
Evidence of cardiomyopathy in hspb7WC3/WC3 adult hearts. Representative images of dissected ventricles and outflow tracts from (A) wildtype and (B) hspb7WC3/WC3 adults. Shown are hearts from 10 month old animals with the atria removed for clear viewing. (C) Quantification of ventricular area to body weight index (VA/BW) showed heart enlargement in animals homozygous for either of the hspb7 mutations. Each point represents one animal. Data are combined for 10 and 18 month zebrafish. Changes in heart size are significant according to an unpaired t-test, corrected for multiple comparisons with the Holm-Sidak method (p<0.0001). (D) Trichrome staining of 3 month wildtype and (E) hspb7 mutant hearts. Focal fibrotic lesions (arrowheads) were seen in all (5/5) hspb7 mutants but never in wildtype samples. Shown are representative images; F and G are higher magnification images for wildtype and mutant hearts, respectively. Arrowheads indicate local fibrotic lesions. (H-K) Representative Trichrome-stained images of adult wildtype (H,J) and mutant (I,K) hspb7WC3/WC3 zebrafish liver (H,I) and skeletal muscle (J,K). |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
Evidence of abnormalities in hspb7WC3/WC3 adult hearts by TEM. Shown are representative transmission electron microscope images of 18 month (A) wildtype and (B) hspb7WC3/WC3 mutant hearts. Wildtype hearts exhibit repeated, regular sarcomeric striations with organized mitochondria. The hspb7 mutant heart tissue shows defects representing breakdown of sarcomeric organization (myofibril packing defects, white arrows). (C and D) Shown are two representative images of hspb7 mutant hearts that also exhibit increased number of autophagosomes (yellow arrowheads). (E) Graphs show quantification of relative occurrence rate (averaged number of defects over the total imaged area) of myofibril packing defects and autophagosomes in the wildtype and hspb7 mutant hearts (n = 3 independent hearts for each). The difference in the rate of each defect was determined using an Exact Poisson test in R. PHENOTYPE:
|
|
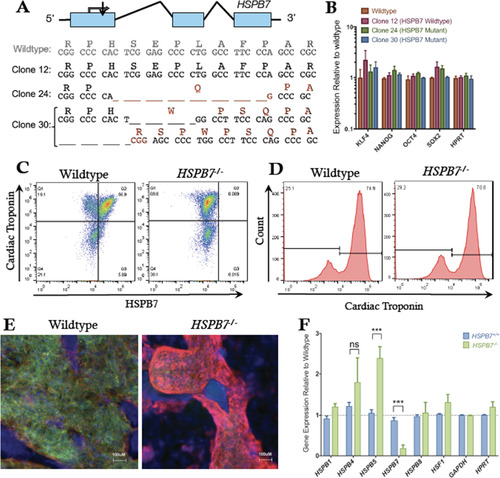
Generation of HSPB7 mutant hESCs. (A) Structure and partial sequence of the human HSPB7 gene, showing CRISPR targeting immediately downstream of the start codon and resultant HSPB7 homozygous and compound heterozygous mutant clones that were recovered. (B) qPCR quantification shows expression levels of pluripotency markers are normal in HSPB7 mutant hESC clones. HSPB7 mutant lines are competent to generate cardiomyocytes, and do not express HSPB7 protein as shown by flow cytometry (C,D) and immunofluorescence (E), both performed on day 10 of differentiation. (F) qPCR quantification of cardiac HSPB family members in wildtype and HSPB7 mutant hESC-derived cardiomyocytes. Changes in gene expression are significant according to an unpaired t-test, corrected for multiple comparisons with the Holm-Sidak method. |
|
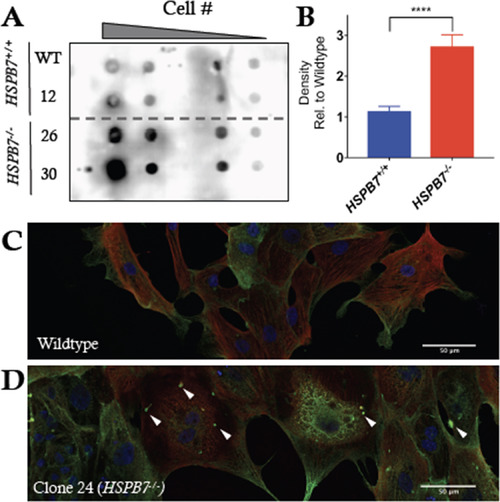
Alteration in FLNC aggregation in HSPB7 mutant CMs. (A) Filter trap assay probing for Filamin-C in lysates from day 10 hESC-derived cardiomyocytes treated with bafilomycin A shows increase in FLNC-containing aggregates in HPSB7-/- cardiomyocytes (lines 26 and 30) compared to wildtype (parental WT and non-targeted line 12). Shown is a representative image from multiple reproducible experiments. (B) Quantification of filter trap assays. Changes are significant according to an unpaired t-test, corrected for multiple comparisons with the Holm-Sidak method (p<0.0001). (C) Immunofluorescence for cardiac troponin (red) and FLNC (green) in hESC-CMs derived from HSPB7 wildtype or (D) HSPB7 mutant lines following treatment with bafilomycin A. White arrowheads mark representative abnormal puncta. |
|
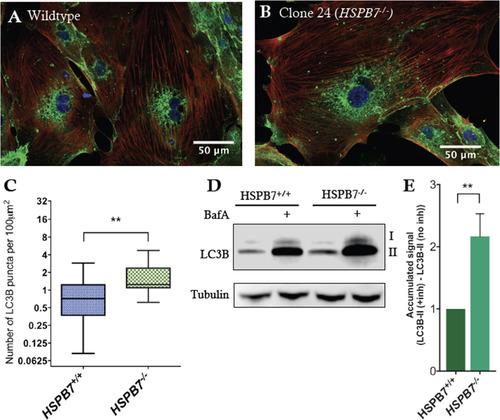
Upregulation of autophagic processes in HSPB7 null cardiomyocytes. Shown are representative images of immunofluorescence for LC3B (green) in (A) wildtype or (B) HSPB7 mutant hESC-derived cardiomyocytes. Samples are co-stained with anti-cardiac troponin T (red) and counterstained with DAPI (blue). (C) Quantification of LC3B puncta in hESC-CMs showing an increase in average number of puncta in HSPB7 mutant cardiomyocytes. (D) Western blot for LC3B in the presence or absence of autophagy inhibitor bafilomycin A (BafA). (E) Quantification of LC3BII western blot by densitometry reveals an increase in the accumulation of LC3BII in HSPB7-/- hESC-CMs during bafilomycin A treatment when compared with HSPB7 wildtype controls. For C and E, changes are significant according to an unpaired t-test, corrected for multiple comparisons with the Holm-Sidak method. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
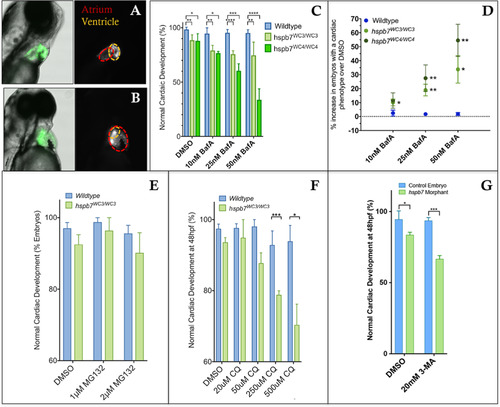
Inhibition of autophagy leads to cardiac malformation in the absence of Hspb7. Shown are representative images of 72 hpf, bafilomycin A (BafA)-treated wildtype (A) or hspb7WC3/WC3 mutant (B) embryos with a tg(myl7: gfp) background showing a cardiac malformation with expansion of the atrium and an undersized ventricle in the hspb7WC3/WC3 fish. (C) Quantification of the percentage of embryos with cardiac abnormalities at 48 hpf, following 24 h treatment with increasing concentrations of Bafilomycin A. n = 40 for each treatment group. (D) hspb7 mutant embryos consistently exhibit significant increases in cardiac abnormalities following treatment with Bafilomycin A. (E) Quantification of cardiac abnormalities in embryos treated with proteasome inhibitor MG132 does not lead to increases in abnormalities in cardiac development in hspb7 mutants. (F) Autophagy inhibitor chloroquine diphosphate (CQ) leads to increases in abnormalities in cardiac development in hspb7 mutants at a greater rate than is seen in wildtype embryos. (G) Treating larvae with the autophagy inhibitor 3-Methyladenine (3-MA) leads to increases in abnormalities in cardiac development in hspb7 morphants at a rate greater than is seen in wildtype control embryos. Abnormalities included small ventricles, unlooped hearts and atrial ballooning. For C, D, F, and G, changes in cardiac abnormalities are significant according to an unpaired t-test, corrected for multiple comparisons with the Holm-Sidak method. |
Reprinted from Developmental Biology, 435(1), Mercer, E.J., Lin, Y.F., Cohen-Gould, L., Evans, T., Hspb7 is a Cardioprotective Chaperone Facilitating Sarcomeric Proteostasis, 41-55, Copyright (2018) with permission from Elsevier. Full text @ Dev. Biol.