- Title
-
Genetic and pharmacological inhibition of CDK9 drives neutrophil apoptosis to resolve inflammation in zebrafish in vivo
- Authors
- Hoodless, L.J., Lucas, C.D., Duffin, R., Denvir, M.A., Haslett, C., Tucker, C.S., Rossi, A.G.
- Source
- Full text @ Sci. Rep.
|
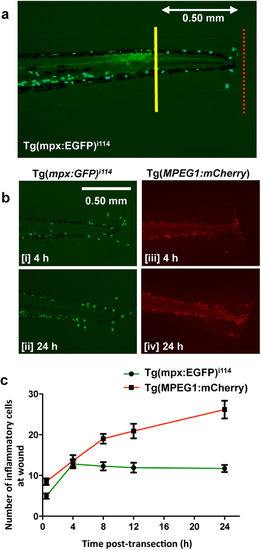
Injury of the zebrafish tailfin results in recruitment of neutrophils and macrophages to the injury site. (a) The tailfin of 3 dpf Tg(mpx:EGFP)i114 and Tg(MPEG1:mCherry) embryos were transected (line of transection shown in red) and a region (0.5 mm length from the tip of the body of the fish) was selected in which to count recruited cells. (b) Temporal recruitment of neutrophils (Tg[mpx:EGFP]i114 [i, ii]) and macrophages (Tg[MPEG1:mCherry] [iii, iv]) post-injury was determined. (c) The numbers of inflammatory cells in each tailfin region were quantified. All time points after 0 h were significantly (p ≤ 0.05) different to the cell numbers at 0 h. All images at 80x magnification. ≥40 fish per group, from 3 independent experiments. Data expressed as ±S.E.M. EXPRESSION / LABELING:
|
|
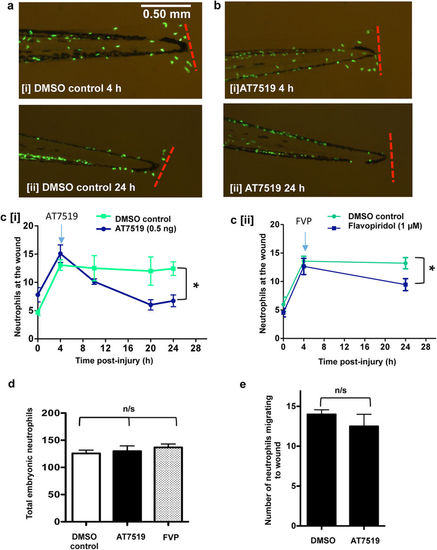
Treatment with AT7519 or flavopiridol accelerates resolution of neutrophilic inflammation. Tg(mpx:EGFP)i114 zebrafish embryos underwent tailfin transection at 3 dpf and were serially imaged at various time points post-injury. (a) Embryos were micro-injected with DMSO or (b) AT7519 at 4 hpi with representative images (80x magnification) from 4 h [i] and 24 h [ii] shown. (c [i]) Serial neutrophil numbers at each tailfin were quantified with the blue arrow indicating the 4 hpi time of drug administration. ≥40 fish from 4 independent experiments. Data expressed as ±SEM. (c [ii]) In separate experiments, Tg(mpx:EGFP)i114 zebrafish embryos were also treated with flavopiridol (FVP) at 4 hpi and the neutrophil numbers at the tailfin quantified. ≥31 fish from 3 independent experiments. (d) The total neutrophils in the entire embryo at 24 hpi and DMSO/AT7519/FVP treatment were imaged and quantified. ≥10 fish from 3 independent experiments. (e) Time lapse movies of embryos (3 per group) were analysed and the number of cells which migrate to the wound for 15 hpi and DMSO/AT7519 treatment was counted. *p < 0.05 at 24 hpi, data analysed by two-way ANOVA then post-test Newman-Keuls or unpaired t-test. n/s: not significant. EXPRESSION / LABELING:
PHENOTYPE:
|
|
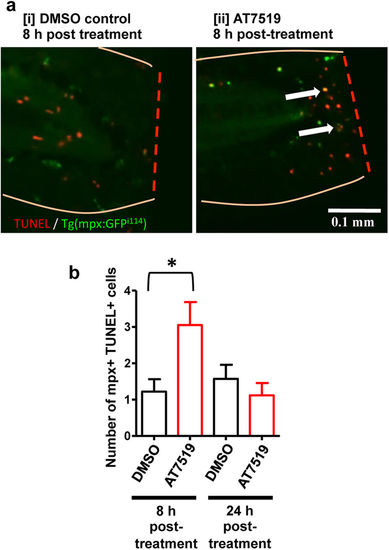
CDK inhibition induces neutrophil apoptosis at the site of tailfin injury in vivo. (a) TSA/TUNEL staining was carried out on Tg(mpx:EGFP)i114 zebrafish following tailfin transection and either DMSO [i] or AT7519 [ii] treatment, labelling neutrophils in green, and apoptotic cells in red, with representative images shown. (b) Apoptotic neutrophils (double positive red and green) are shown by the arrows. The absolute number of double positive cells were quantified at 12 hpi. ≥16 embryos per group in 3 independent experiments. *p < 0.05 12 h DMSO vs 12 h AT7519, unpaired t test. n/s: not significant. Data expressed as ±S.E.M. EXPRESSION / LABELING:
PHENOTYPE:
|
|
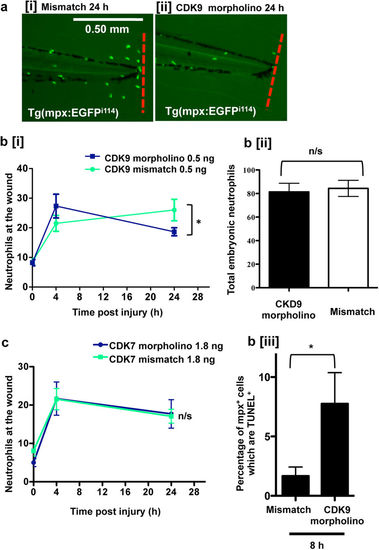
CDK9 knockdown, but not CDK7 knockdown, reduces neutrophilic inflammation. Tg(mpx:EGFP)i114 zebrafish eggs were injected with a CDK9-targeting morpholino or mismatched control sequence and raised to 3 dpf. Embryos with good morpholino uptake were screened, and underwent tailfin transection prior to serial imaging of the wound. (a) Representative images of the tailfin from 24 hpi in mismatch [i] or morpholino sequence-injected fish [ii] are shown, with cumulative data (b [i]). n ≥ 38 fish from 4 independent experiments. (b) The total neutrophils in the whole embryo at 3 dpf were counted [ii] and the percentage of apoptotic neutrophils at 8 hpi was calculated by quantifying TSA+/TUNEL+ double positive cells as a percentage of total TSA+ cells [iii]. Tg(mpx:EGFP)i114 zebrafish eggs were similarly injected with a CDK7-targeting morpholino or mismatched control sequence. (c) The tailfin was transected at 3 dpf and imaged at 0, 4 and 24 hpi with cumulative data shown. ≥16 fish per group in 3 independent experiments. *p < 0.05, n/s: not significant, analysed two-way ANOVA followed by post-hoc Newman Keuls test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
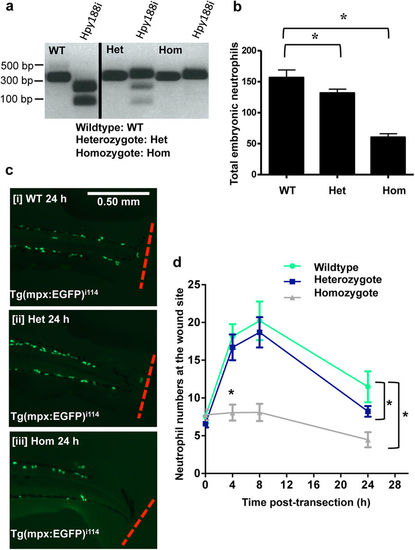
CRISPR/cas9 knockout of CDK9 reduces neutrophilic inflammation. Tg(mpx:EGFP)i114 zebrafish eggs were micro-injected with cas9 mRNA and a guide mRNA to target CDK9. (a) These animals were raised and in-crossed, then PCR and restriction digests with the restriction enzyme Hpy188i were carried out to assess if embryos were homozygote (Hom), wild type (WT) or heterozygous (Het). The black dividing line is used to show this is two example images cropped from different areas of the same gel. (b) Total embryonic neutrophils in fish from each group of embryos were counted. (c,d) The tailfin of WT, Hom and Het embryos were transected and the neutrophils at the site of wounding were imaged at various time points and quantified. ≥47 fish per group from 3 independent experiments. Data shown as ±S.E.M. *p < 0.05, n/s: not significant, assessed by two-way ANOVA followed by post-hoc Newman Keuls test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
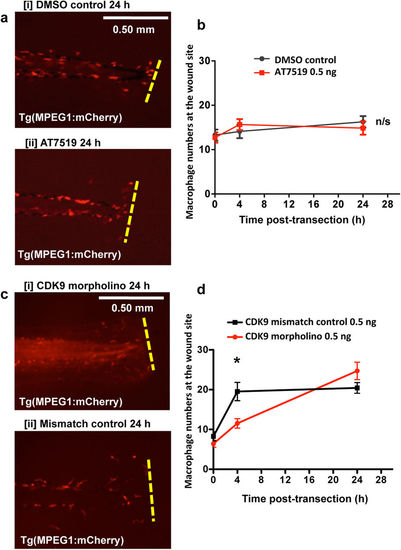
AT7519 and CDK9 morpholino knockdown has no effect on macrophage accumulation post-injury. Tg(MPEG1:mCherry) zebrafish underwent tailfin transection and were treated with DMSO (control) or AT7519 at 4 hpi. (a) Example images of DMSO [i] and AT7519 [ii] – treated animals at 24 hpi are shown with cumulative data in (b). ≥22 fish per group over 3 independent experiments. Newly laid Tg(MPEG1:mCherry) zebrafish eggs were also injected with a CDK9 splice-blocking morpholino or mismatched control sequence, then raised to 3 dpf. (c) The embryo tailfin was transected and the fish imaged at various time points with example images shown at 24 hpi for morpholino [i] and control [ii] fish and cumulative data shown in (d). n ≥ 20 fish from 3 independent experiments. Data shown as ±S.E.M. *p < 0.05, n/s: not significant, assessed by two-way ANOVA followed by post-hoc Newman Keuls test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
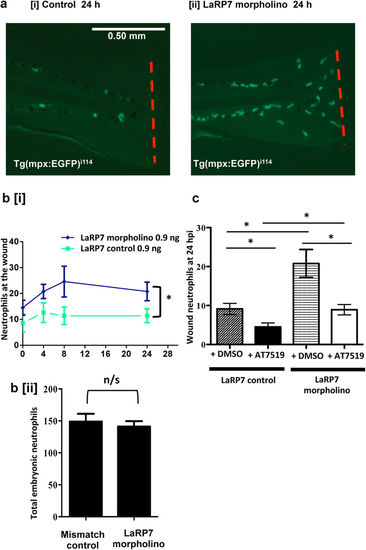
Knockdown of LaRP7 increases inflammation at the wounded tailfin, and this can be inhibited using the CDK inhibitor AT7519. (a) Tg(mpx:EFGP)i114 zebrafish eggs were injected with a mismatched control or a morpholino sequence to knockdown LaRP7, and at 3 dpf the tailfin was transected with representative images at 24 hpi shown [i, ii]. (b) Neutrophils at the wounded tailfin [i] and also in the whole embryo [ii] were quantified. ≥10 fish per group in 3 independent experiments. (c) LaRP7 knockdown and control zebrafish were also injected with AT7519/DMSO at 4 hpi and the neutrophils quantified at various time points; shown is the 24 hpi time point. ≥19 fish per group in 3 independent experiments. Data shown as ±S.E.M. *p < 0.05. n/s: not significant. Data analysed by two-way ANOVA followed by post-hoc Newman Keuls or unpaired t-test. |