- Title
-
Structure of the polyisoprenyl-phosphate glycosyltransferase GtrB and insights into the mechanism of catalysis
- Authors
- Ardiccioni, C., Clarke, O.B., Tomasek, D., Issa, H.A., von Alpen, D.C., Pond, H.L., Banerjee, S., Rajashankar, K.R., Liu, Q., Guan, Z., Li, C., Kloss, B., Bruni, R., Kloppmann, E., Rost, B., Manzini, M.C., Shapiro, L., Mancia, F.
- Source
- Full text @ Nat. Commun.
|
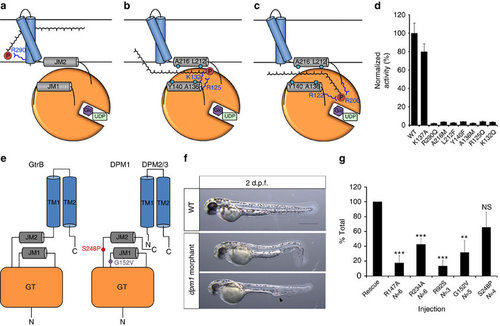
A putative mechanism for substrate translocation in GtrB and DPM1 function in a zebrafish model. (a) A speculative hypothesis is that the phosphate headgroup of UndP (red) could first bind at R290, near the cytoplasmic face of the inner membrane. (b) The substrate could then diffuse along a pathway lined with conserved hydrophobic (teal) and positively charged (blue) residues. (c) Finally, the phosphate headgroup is coordinated by R122 and R200 at the acceptor site, where catalysis occurs. (d) Mutation of hydrophobic and positively charged conserved residues lining the region between JM1 and JM2 abrogates GtrB activity. Error bars are provided as s.e.m., n=3. (e) The architecture of GtrB may be shared by the DPMS complex. CDG1e mutations G152V and S248P in the conserved JM1 and JM2 linkers are represented in purple and red, respectively. (f) Phenotypes typical of dpm1 loss-of-function mutations are fully evident at 2 days post fertilization (d.p.f.) in the zebrafish embryo. Morphants show smaller head (microcephaly) and smaller eyes, kinked tail and occasional vascular defects in the tail vein (arrowhead). Scale bar, 500 µm (g) Functional analysis of DPM1 mutants was performed by injecting human DPM1 mRNA following dpm1 morpholino injection and embryos were scored as normal or affected. Human DPM1 mRNA was able to improve the morphant phenotypes (see Supplementary Fig. 8f for details) and the effect of different mutations was normalized to rescue levels. mRNA carrying the R147A or R234A mutations (equivalent to R122 and R200 in GtrB, respectively) showed almost complete loss of function. Known human missense changes (R92S, G152V and S248P) were also tested. R92S and G152V greatly abolished protein function, whereas S248P, which leads to milder human phenotypes, showed substantial residual activity. The presumed location of G152V and S248P on the juxtamembrane region is shown. R92S is expected to reside close to the active site in the GT domain. Error bars: s.e.m., **P<0.01, ***P<0.001, unpaired Student’s t-test for each mutant, n=6 (R147A), n=6 (R234A), n=3 (R92S), n=5 (G152V), n=4 (S248P). NS, not significant. PHENOTYPE:
|
|
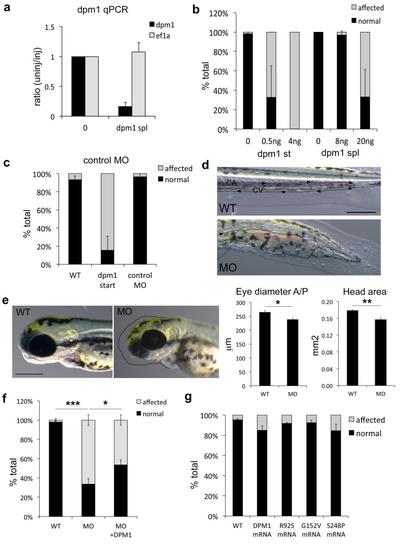
dpm1 knockdown in zebrafish recapitulates the human phenotypes caused by DPM1 mutations. Multiple controls to confirm the effectiveness and specificity of the dpm1 morpholinos (MOs) were performed. All error bars represent S.E.M. *=p<0.05, **=p<0.01, ***=p<0.001, unpaired student’s t-test, N’s are indicated for each experiment. (a) Knockdown efficiency was measured in affected embryos using 10ng of dpm1 splice MO at 1dpf. No difference is observed in expression of the housekeeping gene ef1a. (N=3) (b) Splice and start dpm1 MOs caused identical phenotypes as shown in Fig. 4 and in this figure in d and e at different concentrations. As start MOs are usually more effective 4ng were sufficient to generate 100% affected embryos at 1dpf, while the splice MO needed to be injected at higher concentrations. (N=3) (c) Injection of a scrambled control morpholino in parallel to maximally effective concentrations of the start MO had no effect on the embryos, showing that these phenotypes are not due to injection and general MO toxicity. (N=4) (d) Vascular defects reminiscent of those observed in the patients can also be occasionally observed in the dpm1 morphant. The caudal artery (CA) and caudal vein (CV) supply blood flow to the tail in the direction indicated by the arrows in the WT panel. In the morphants blood vessels appear fused and enlarged (outlined by the dotted line) leading to accumulation of blood in the distal portion of the tail. Scale bar: 200µm. (e) Microcephaly, a reduction in head size, and a reduction in eye size are observed in most morphants. Dotted lines in the morphants (MO) panel shows the outline of the wild-type (WT) head in black and of the WT eye in white for reference. Scale bar: 200µm. Quantification of the reduced eye diameter measured on the anterior/posterior (A/P) axis and of head area is shown (N=3). (f) Rescue experiments were performed using 2ng dpm1 start MO injections as described in the Methods and 200pg of DPM1 mRNA, then the number of normal vs. affected embryos were tested. Despite embryos and larvae in the rescue condition appeared less affected than MO injected clutchmates, this difference was not clearly quantifiable due to the multiple phenotypes observed, so the total number of normal vs. affected was used (N=6). (g) To rule out possible gain of function effects of human missense mutations, normal and mutated DPM1 mRNAs were injected in isolation and none caused substantial defects (N=3). PHENOTYPE:
|