- Title
-
nlz1 Is required for cilia formation in zebrafish embryogenesis
- Authors
- Dutta, S., Sriskanda, S., Boobalan, E., Alur, R.P., Elkahloun, A., Brooks, B.P.
- Source
- Full text @ Dev. Biol.
|
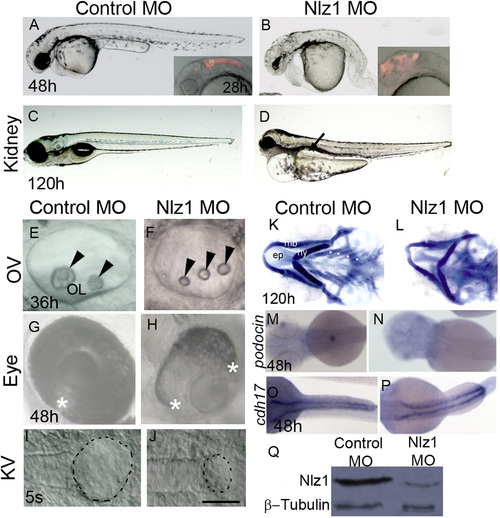
Nlz1 morpholino-injected embryos show morphological defects similar to a human ciliopathy. (A)–(J) DIC images of live embryos at different developmental stages (A), (B), (G), and (H) 48 h; (C), and (D) 120 h; (E), (F) 36 h; (I), and (J) 5S. Embryos were injected with Control MO (A), (C), (E), (G), and (I) or with Nlz1 MO (B), (D), (F), (H), (J) at the 1–2 cell stage. Nlz1 MO injected embryos show generalized edema; an abnormal brain; a short, bent tail (B); kidney cyst(s) (D, arrow, n=45/55); three small otoliths in the OV (F, arrow head, n=52/60); and a rudimentary KV (J, dotted line, n=15/40). In control MO injected embryos, gross morphology (A), kidneys (C), otoliths (E), and KV (I) were normal. Control MO injected embryos (A, inset), and nlz1 morphants (B, inset) were injected with rhodamine dextran in the hindbrain ventricles at 28 h.The two edges of the optic fissure were fused completely in control MO injected embryos (G, asterisk, n=50/50) whereas coloboma was observed in nlz1 morphants (H, n=72/80). (K)–(L) Alcian blue staining of pharyngeal cartilages in control MO (K) and Nlz1 MO (L) injected zebrafish larvae at 120 h. (M)–(P) Dorsal view of a whole mount in situ hybridization with probes for podocin (M), and (N), and cdh17 (O), and (P) in control MO (M), and (O) and Nlz1 MO (N), and (P) injected embryos at 48 h. (Q) Western blot shows severe reduction of Nlz1 protein expression in morphants. (B), (F), (H), and (J) injected with 3.5 ng Nlz1 MO, (D), (L), (N), and (P) injected with 2 ng Nlz1 MO. (A)–(H) lateral views, anterior to the left; (I)–(J) dorsal view, posterior to the top. Scale bar in (A) and (B), and insets, 320 µm; (C) and (D), 200 µm; (E) and (F), 35 µm; (G) and (H), 100 µm; (I) and (J), 50 µm. OV, otic vesicle; KV, Kupffer’s vesicle; OL, otolith; s, somite; ep, ethmoid plate; mb, mandibular arch; hy, hyoid arch; asterisks indicate branchial arches. EXPRESSION / LABELING:
PHENOTYPE:
|
|
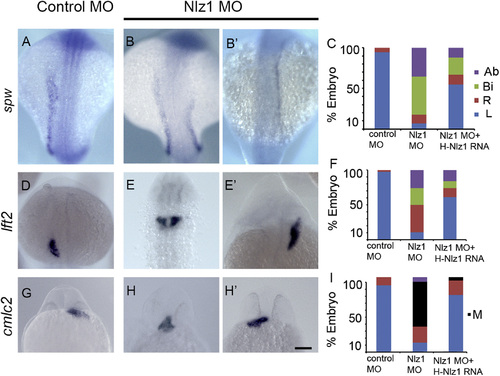
Nlz1 is important for left–right patterning in zebrafish. Zebrafish embryos were injected with control MO (A), (D), and (G) or with 2 ng of Nlz1 MO (B), (B2), (E), (E′), (H), and (H′). Embryos were hybridized in situ with laterality marker genes spw (A), (B), (B′), lft2 (D), (E), and (E2), and cmlc2 (G), (H), (H′) RNA probes. Quantification of expression patterns of spw (C), lft2 (F), and cmlc2 (I) in MO (spw, n=85; lft2, n=110; cmlc2, n=75) and MO+mRNA injected embryos (spw, n=75; lft2,n=100; cmlc2, n=55). (A), (B), (B′), (D), (E), (E′) dorsal view at 18–20S stage, (G), (H), (H′) frontal view at 28 h. Scale bar 100 µm. Ab: absent; Bi: bilateral, M: midline; R: right; L: left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
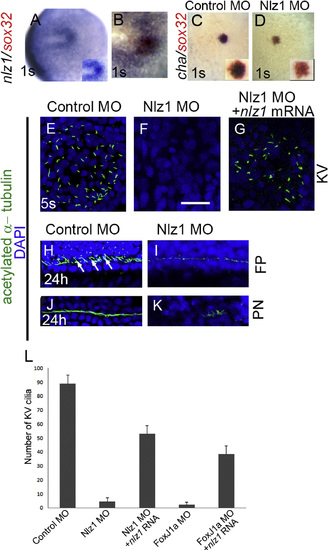
nlz1is expressed in Kupffer’s vesicle and is required for cilia formation in zebrafish. Whole mount in situ hybridization at the one somite (1S) stage with one or two RNA probes as indicated in wild type (A), and (B), control MO injected (C), and Nlz1 MO injected (D) embryos. (B) Cells in the KV co-express nlz1 and sox32, marking dorsal forerunner cells. In nlz1 morphants cha (D, n=28/35) was absent in the KV region, whereas sox32 (red) remained unaltered as compared to control MO injected embryos (C). Cilia were marked with acetylated-α tubulin (green) and nuclei were counterstained with DAPI (blue) in different regions of the embryos as indicated. Complete absence of KV cilia was observed in nlz1 morphants (F, n=15/18) compared to control (E, n=15/15). Similarly, at later stages, the number and length of the cilia in the neural FP (I, n=18/23) and in the PN (K, n=27/30) were reduced in nlz1 morphants compared to control MO injected embryos (H), and (J). Arrows indicate motile cilia. KV cilia in nlz1 morphants were rescued by nlz1 mRNA injection (G, n=45/52). (L) Quantification of cilia number in KV embryos injected with MO±mRNA. (A), and (B) dorsal view, anterior to left; (C) and (D) dorsal view, posterior to top. (D), (F) and (G) injected with 2.5 ng Nlz1 MO, (I), and (K) injected with 2 ng Nlz1 MO. Inset (A), (C), and (D) shows blow up of KV region. KV; Kupffer’s vesicle; s, somite; FP, floor-plate; PN, pronephros. Scale bar in (A)–(D), 100 µm; (E)–(G), 12.5 µm; (H)–(K), 50 µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) EXPRESSION / LABELING:
PHENOTYPE:
|
|
Nlz1 is downstream of Foxj1a and is regulated by canonical Wnt signaling. (A)–(D), (A′)–(D′) nlz1 expression in foxj1a morphants (B, B′, n=48/50), wnt8a morphants (C, C′, n=52/55), and LiCl treated foxj1morphants embryos (D, D′, n=25/28) compared to control MO (A, A′, n=30/30), as seen in whole mount in situ hybridization of embryos at 1S stage. (E)–(F) Cilia are labeled with anti-acetylated α tubulin antibody (green) in the KV at the 5S stage. Complete lack of KV cilia was observed in foxj1a morphants (E, n=20/20); injection of nlz1 mRNA in foxj1a morphants rescues KV cilia (F, n=12/30). Quantification of expression patterns of cmlc2 (G), and lft2 (H) in Foxj1a MO (cmlc2, n=50; lft2, n=50) and Foxj1a MO+nlz1mRNA (cmlc2, n=50; lft2, n=50) injected embryos. (A)–(F) dorsal view, (A)–(D) anterior dorsal, (A′)–(D′) posterior dorsal, (E), and (F) posterior to the top. (H), (J) Embryos were treated with LiCl as specified. (I)–(L) Whole mount in situ hybridization at 95% epiboly (I), (J) and 21 h (K), (L) resulted in expansion of nlz1 expression (J, n=45/50; L, n=15/19) compared to control (I n=40,K n=40, without LiCl). (I), (J) dorsal view, (K), (L) lateral view, anterior to left. (M) Nlz1 repressed Wnt/β-catenin signaling activated by either LiCl or β-catenin in a TOPflash luciferase assay. RLU, relative luciferase units. Ab: absent; Bi: bilateral, M: midline; R: right; L: left. Scale bar (black) in (A)–(D′), 100 µm; (I)–(J), 60 µm; (K)–(L), 75 µm. Scale bar (white) in (E)–(F), 50 µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) EXPRESSION / LABELING:
PHENOTYPE:
|
|
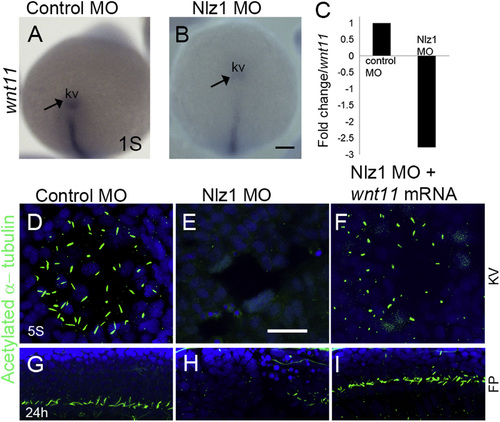
wnt11 rescues cilia defects innlz1morphant fish (A), and (B) Whole mount in situ hybridization at 3S stage with wnt11 antisense RNA probe as indicated in (A) control MO injected, and (B) Nlz1 MO injected embryos. (C) Bar diagram demonstrating down-regulation of wnt11 expression (~2.5 fold ) in nlz1 morphant embryos compare to control. (D)–(F) Cilia were labeled with anti-acetylated tubulin in KV, and (G)–(I) in FP. Motile cilia were absent in KV and FP of nlz1 morphants (E, n=28/32, H, n=17/24), compared to control MO injected embryos (D, n=40/40, G, n=40/40). Co-injection of wnt11 mRNA and Nlz1 MO (F, n=20/41, I, n=25/41) partially rescues cilia in KV and the FP. (A), and (B) posterior view. KV; Kupffer’s vesicle; s, somite; FP, floor-plate. Scale bar A and B, 100 µm; (D)–(F), 12.5 µm; and (G)–(I), 50 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
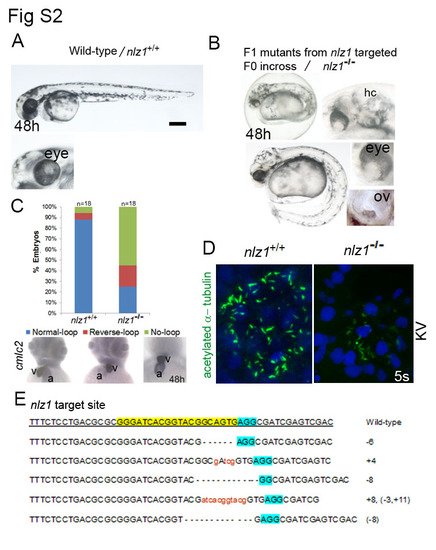
CRISPR/Cas9 mediated nlz1 knockout alters heart laterality and motile cilia formation (A) Wild-type embryo at 48h. After putative F0 founders (3 male and 3 female) were bred in one tank, the F1 nlz1 mutants (B, n=20/150) had bent tail, hydrocephaly, ocular coloboma, and three otoliths in the otic vesicle. (C) in situ hybridization using cmlc2 ribo probe showed different heart looping ( normal looping, reverse looping, no looing) phenotypes in zebrafish embryos at 48h. Bar graph represent distribution of different heart looping phenotypes in wild-type and in nlz1-/- mutant embryos. (D) Motile cilia in the KV were labelled with acetylated -α-tubulin antibody (green), nuclei were counterstained with DAPI (blue). In nlz1-/-mutant embryos (n=5/5) the number of KV cilia were dramatically reduced compared to nlz1+/+ wild-type embryos (n=10/10). (E) Sequences of the nlz1 target region in eight F1 putative mutant progeny. All sequences had indels close to nlz1 target region (highlighted in yellow), and 5 different nlz1 mutant alleles were recovered. The sequence variations are indicated in the right (+ insertion, - deletion), the wild-type reference allele sequence is underlined, and the protospacer- adjacent motif (PAM) is highlighted in blue. (A,B) Lateral view, anterior to left; (C) anterior view; (D) flat mount of posterior region; a, atrium; v, ventricle; hy, hydrocephaly; 1 ov, otic vesicle;KV, Kupffer’s Vesicle; scale bar 100 µm. PHENOTYPE:
|
|
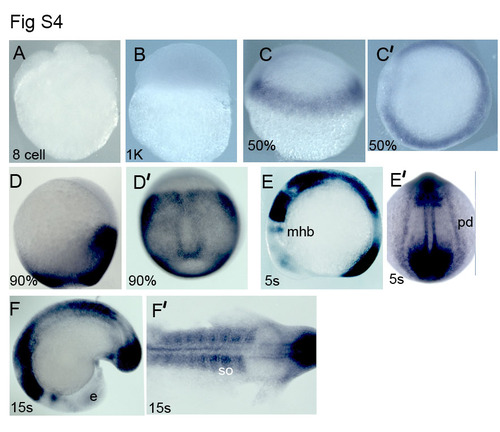
nlz1 expression pattern in zebrafish embryos (A-F′)Whole mount in situ hybridization of zebrafish embryos with nlz1 riboprobe at different developmental stages as indicated at the lower bottom of each panel. mhb, mid-hindbrain boundary; e,eye; pd, pronephric duct. (C′, D′, E′) dorsal view; (E, F, F′) lateral view. EXPRESSION / LABELING:
|
|
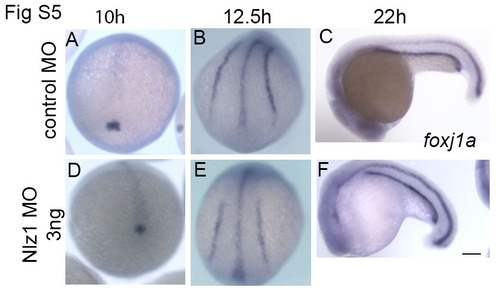
foxj1a expression is unaltered in nlz1 morphant embryos (A-C) Control MO injected, and (D-F) Nlz1 MO injected embryos were in situ hybridized with foxj1a antisense RNA probe. The stages of embryos are indicated on the top. Scale bar 50µm. (A,B,D,E) dorsal view; (C,F) lateral view. |
|
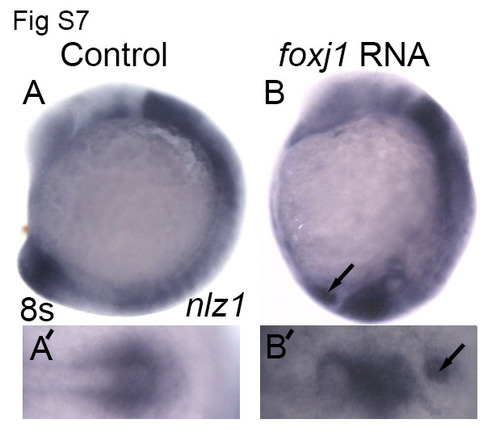
foxj1a induce ectopic nlz1 expression (A, A′) control embryos and (B, B′) foxj1 mRNA injected embryos were fixed in PFA at 8- somite stage and nlz1 expression was assayed by whole mount in-situ hybridization . Arrows indicate ectopic expression of nlz1 (B, B′, n=28/35) in the posterior region of the embryo. (A, B) lateral view, anterior to top; (A′, B′) posterior view. |
Reprinted from Developmental Biology, 406(2), Dutta, S., Sriskanda, S., Boobalan, E., Alur, R.P., Elkahloun, A., Brooks, B.P., nlz1 Is required for cilia formation in zebrafish embryogenesis, 203-11, Copyright (2015) with permission from Elsevier. Full text @ Dev. Biol.