- Title
-
Mutations in MAB21L2 Result in Ocular Coloboma, Microcornea and Cataracts
- Authors
- Deml, B., Kariminejad, A., Borujerdi, R.H., Muheisen, S., Reis, L.M., Semina, E.V.
- Source
- Full text @ PLoS Genet.
|
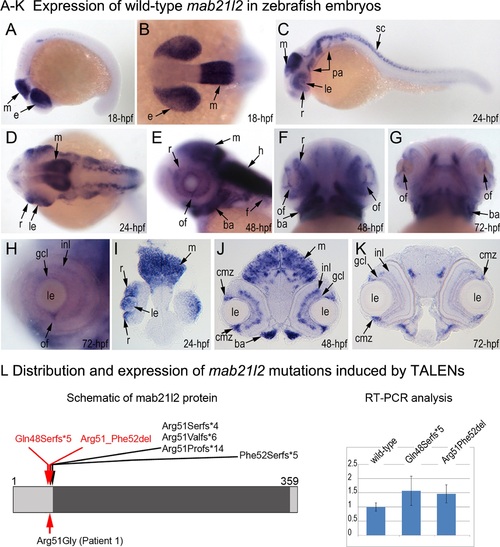
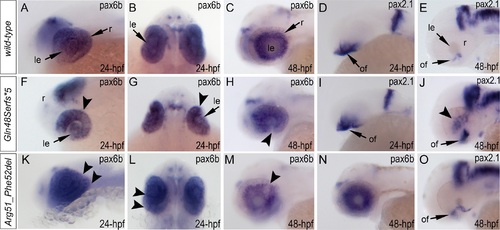
Expression and mutations of zebrafish mab21l2. A-K. Expression pattern of mab21l2 in zebrafish 18–72-hpf embryos. Whole mount images (A-H) and sections (I-K) are shown. A, B. At 18-hpf, expression in the presumptive eye field (e) and midbrain (m) is observed. C, D, I. At 24-hpf, mab21l2 expression is seen in the periphery of the retina (r), lens (le), spinal cord (sc), midbrain (m) and pharyngeal arch region (pa). E-H, J, K. At 48–72-hpf, expression in the ciliary marginal zone (cmz), inner nuclear layer (inl) and ganglion cell layer (gcl) of the retina, and the region of the optic fissure (of) in the eye as well as the midbrain (m), hindbrain (h), developing fins (f), and branchial arches (ba) is shown with arrows. L. Distribution and expression of mab21l2 mutations induced by TALENs. On the left, a schematic of the zebrafish mab21l2 protein is shown as a light grey box with the mab-21 domain (amino acids 62–346) indicated in dark grey color; the positions of the zebrafish mutations identified in the progeny of TALEN-injected fish are shown at the top of the box and the position of the human mutation identified in Patient 1 is indicated at the bottom; the positions of the p.(Gln48Serfs*5) and p.(Arg51_Phe52del) mutations are shown with red arrows. On the right, a graph summarizing results of semi-quantitative RT-PCR analysis of wild-type and mutant mab21l2 transcript levels in 48-hpf homozygous embryos is shown. EXPRESSION / LABELING:
|
|
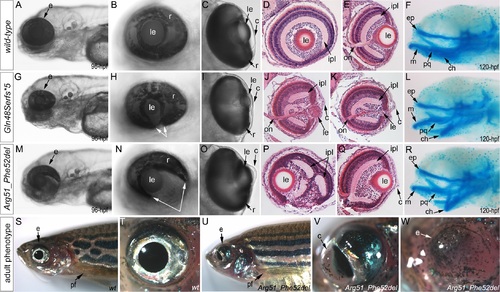
Phenotypic analysis of homozygous embryos carrying mutant mab21l2 alleles encoding p.(Gln48Serfs*5) truncation (mab21l2Q48Sfs*5) and p.(Arg51_Phe52del) in-frame deletion (mab21l2R51_F52del) proteins. A-R. Embryonic phenotypes. Images of wild-type larvae at 96-hpf (A-E) and 120-hpf (F), as well as embryos carrying the p.(Gln48Serfs*5) frameshift (G-L) or p.(Arg51_Phe52del) in-frame deletion (M-R) alleles are shown. Whole mount images (A-C, G-I, and M-O), as well as frontal (E, J, K, Q) and sagittal (D, P) ocular sections are presented. Please notice reduced eye size (G-K), coloboma (white arrows in H), degenerative (H-J) or absent (K) lens, disorganized retina and irregular cornea (J, K) in embryos with frameshift mutations, as well as severe coloboma (white arrows in N) with disorganized retina, discontinuous RPE (white arrows in P, Q), and corneal defects (O) but overall comparable to wild-type eye size and lenses (M-Q) in embryos that are homozygous for the in-frame deletion. Alcian blue stain of wild-type (F) and mutant embryos (L, R) identified defects in craniofacial development with primary defects in the development of the ceratohyal cartilage of the hyoid arch (or the second pharyngeal arch). S-W. Adult phenotype. Images of adult wild-type (S, T) and Arg51_Phe52del mutant (U-W) fish: please notice microphthalmic highly disorganized eye with pigmented cornea (V) and anophthalmic contralateral eye with residual abnormal pigmented tissue (W). Please also note normal appearance of pectoral fins in the mutant fish (U). ch, ceratohyal; ep, ethmoid plate; m, Meckel′s cartilage; pq, palatoquadrate; e, eye; c, cornea; le, lens; ipl, inner plexiform layer; on, optic nerve; pf, pectoral fins; r, retina. |
|
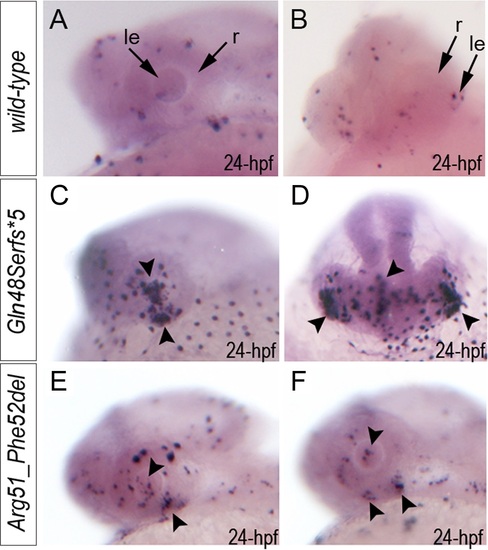
Summary of TUNEL assays in zebrafish wild-type and mab21l2 mutant embryos. TUNEL results in 24-hpf wild-type (A-B), mab21l2Q48Sfs*5 embryos (C-D) and mab21l2R51_F52del mutants (E-F) are shown. An increase in TUNEL staining was observed in both mab21l2 mutants with remarkably high levels in the mab21l2Q48Sfs*5 embryos, particularly in the lens and ventral retina (C-D), and moderately increased levels in the mab21l2R51_F52del embryos (E-F); arrowheads indicate sites of increased TUNEL staining in the eye and brain; le, lens; r, retina. PHENOTYPE:
|
|
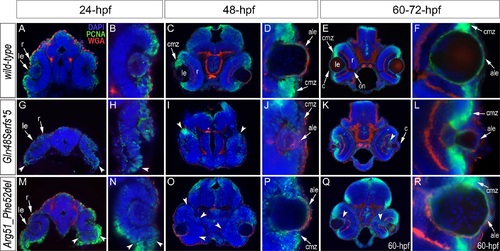
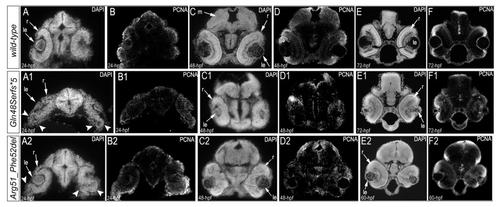
Analysis of proliferation patterns and differentiation markers in wild-type and mutant embryos. Immunostaining with PCNA (Proliferating Cell Nuclear Antigen) (green), DAPI (blue), wheat germ agglutinin (WGA) (red) was performed using 24–72-hpf wild-type embryos (A-F) as well as mab21l2Q48Sfs*5 (G-L) and mab21l2R51_F52del (M-R) mutant tissues. Embryonic stages are indicated above; overlay fluorescence images are shown and single immunoreactivity data is available in S4 Fig. White arrowheads point to defects in retinal invagination and regions of abnormal retinal folding (H,M,N) observed in both mab21l2Q48Sfs*5 and mab21l2R51_F52del embryos (please see text), as well as areas of aberrant PCNA labeling (I,K,O,Q). Please also note the absence of PCNA staining in the anterior lens epithelium of 48- and 72-hpf mab21l2Q48Sfs*5 mutants (J,L). ale, anterior lens epithelium; c, cornea; cmz, ciliary marginal zone; le, lens; on, optic nerve; r, retina. |
|
Analysis of pax6b, pax2.1 and foxe3 expression in wild-type, mab21l2Q48Sfs*5 and mab21l2R51_F52del embryos. Wild-type (A-E) and mutant (F-O) zebrafish embryos at 24–48-hpf were analyzed as indicated in the right bottom corner of each image. Please note a change in pax6b transcript distribution at 24-hpf and 48-hpf in mab21l2Q48Sfs*5 (arrowheads in F-H), retinal folding defect in mab21l2R51_F52del embryos at 24-hpf (arrowheads in K, L) and visibly abnormal pax6b pattern at 48-hpf in some (arrowheads in M) but not all (N) mab21l2R51_F52del embryos. pax2.1 expression seems to be unaffected in 24-hpf frameshift mutant embryos (I) but shows an abnormal pattern in both mutants at 48-hpf (J,O). At 48-hpf, in addition to more broad and intense pax2.1 expression in the region of optic fissure, abnormal pax2.1 staining was detected in central retina in mab21l2Q48Sfs*5 embryos (arrowheads in J). le, lens; of, optic fissure; retina. |
|
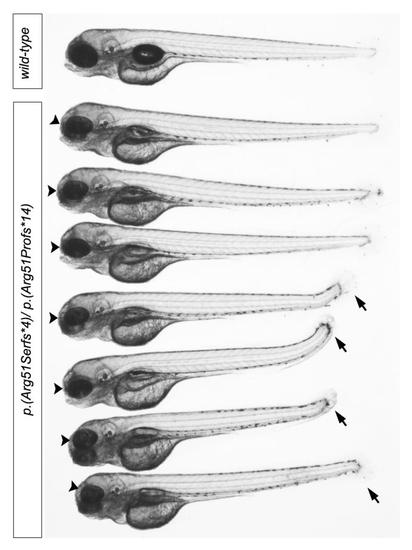
mab21l2 compound heterozygous embryos generated by the cross of c.150_156delCCGTTTC, p.(Arg51Serfs*4) and c.151dupC, p.(Arg51Profs*14) heterozygous carriers. A wild-type fish from the same progeny is shown at the top and followed by 7 compound heterozygous embryos. Please note small eye (black arrowheads) in all mutant embryos and shortened tail (black arrows) in 4 out of 7 fish. |
|
TUNEL results in 48–72-hpf wild-type (A-D), mab21l2Q48Sfs*5 embryos (E-H) andmab21l2R51_F52del mutants (I-L) are shown. An increase in TUNEL staining was observed in both mab21l2 mutants with remarkably high levels in the mab21l2Q48Sfs*5 embryos (E-H) and moderately increased levels in themab21l2R51_F52del embryos (I-L); arrowheads indicate sites of increased TUNEL staining in the eye and brain; le, lens; r, retina; m, midbrain. PHENOTYPE:
|
|
Immunostaining with PCNA (Proliferating Cell Nuclear Antigen) and DAPI in 24–72-hpf wild-type (A-F), mab21l2Q48Sfs*5 (A1-F1), and mab21l2R51_F52del (A2-F2) embryos. Overlay images of the PCNA and DAPI immunostaining are shown in Fig. 6. The arrowheads in A1 and A2 indicate abnormal retinal folding;le, lens; m, midbrain; r, retina. |
|
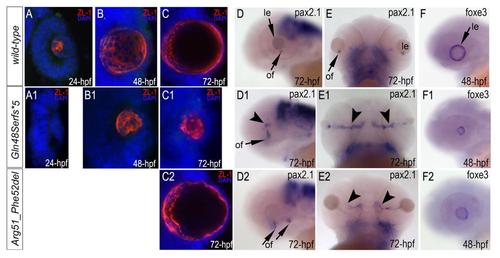
Immunostaining with ZL-1 (red) and in situ hybridization with pax6b, pax2.1 and foxe3 antisense riboprobes in wild-type (A-F), mab21l2Q48Sfs*5 (A1-F1) andmab21l2R51_F52del (C2-F2) embryos. The ZL-1 staining is absent in 24-hpf (A1) but present in 48–72-hpf mutant embryos (B1, C1, C2), pax2.1 pattern is abnormal in 72-hpf mutant embryos (D1, E1, D2, E2), arrowhead in D1 shows abnormal areas of pax2.1-positive cells in the central retina and arrowheads in E1 show broad and intense expression in the region of optic fissure; foxe3 expression is decreased in 48-hpf mutants (F1, F2); le, lens; of, optic fissure. EXPRESSION / LABELING:
|