- Title
-
Systematic classification of vertebrate chemokines based on conserved synteny and evolutionary history
- Authors
- Nomiyama, H., Osada, N., and Yoshie, O.
- Source
- Full text @ Genes Cells
|
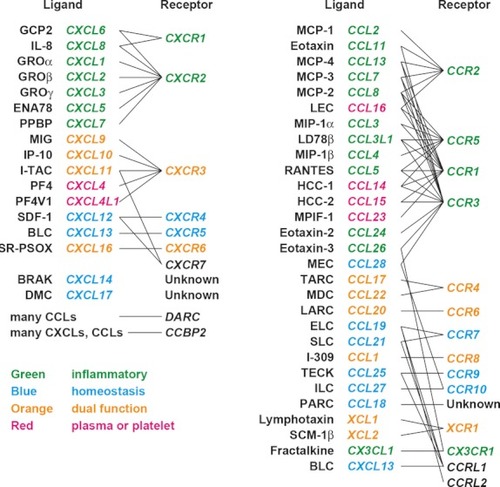
Chemokine ligand–receptor binding relationships. Five subfamilies of chemokines, CXC, CC, XC, CX3C, and CX, have been recognized on the basis of the arrangement of the two N-terminal residues of four conserved cysteines. One and three amino acids separate the first and second cysteines in the CXC and CX3C chemokines, respectively, whereas the two cysteines are adjacent to each other in the CC subfamily. The XC (or C) subfamily lacks the first and paired third cysteine residues. The fifth subfamily, CX, which has so far been identified only in zebrafish, lacks one of the two N-terminal cysteine residues but retains the third and fourth ( |
|
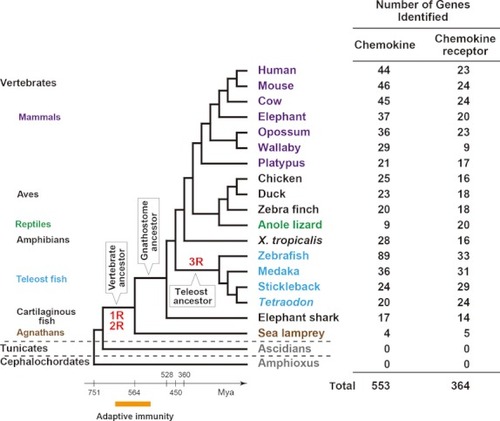
Number of chemokine and chemokine receptor genes identified in vertebrate genomes. We had previously identified chemokine genes from 10 mammalian genomes ( |
|
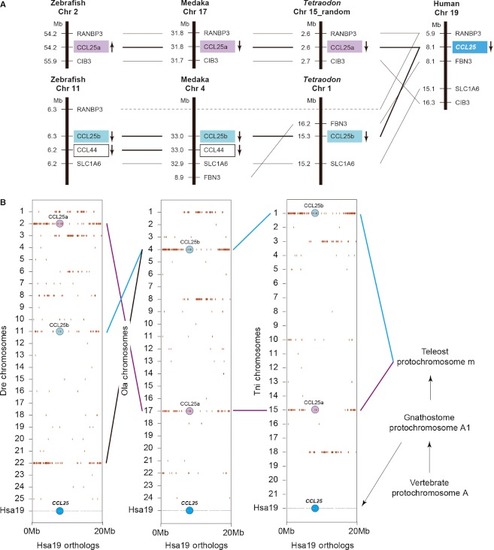
Conserved synteny analysis of vertebrate chemokine |
|
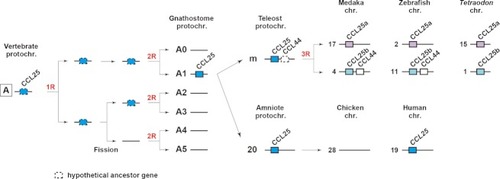
Proposed ancestry of vertebrate chemokine |
|
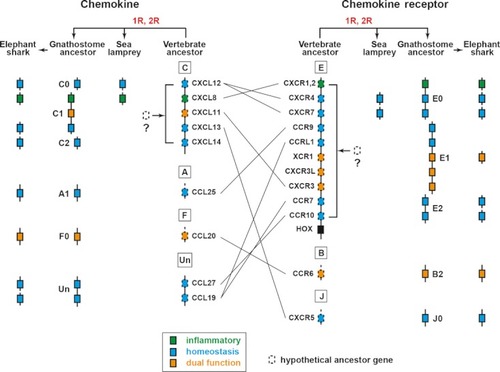
Vertebrate ancestral genes for chemokines and chemokine receptors. The vertebrate and gnathostome protochromosomes on which chemokine and chemokine receptor genes were localized are shown. Among the genes contained by sea lamprey and elephant shark, only those that are shared by vertebrate or gnathostome ancestors are shown. Because the genome sequences of sea lamprey and elephant shark are still fragmented, it is not known whether the genes are linked on the same chromosomes. The lines that link the vertebrate chemokine ancestors with the chemokine receptor ancestors indicate the ligand–receptor relationships based on the human chemokine system. The receptor for |