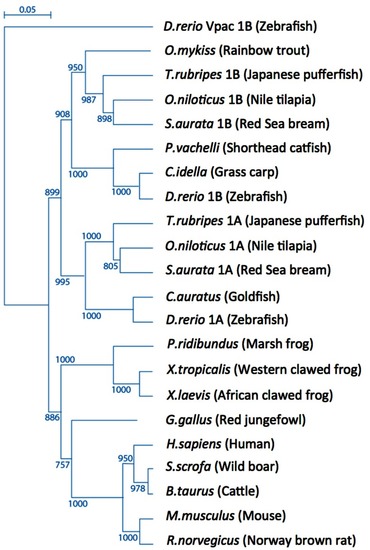
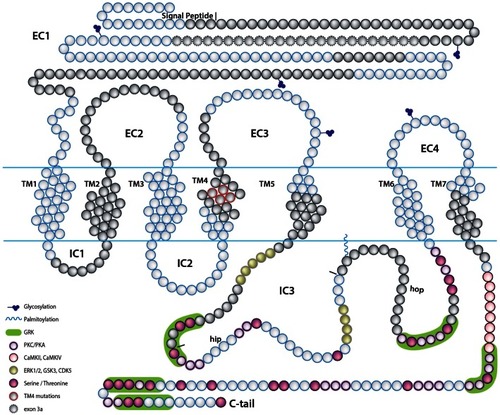
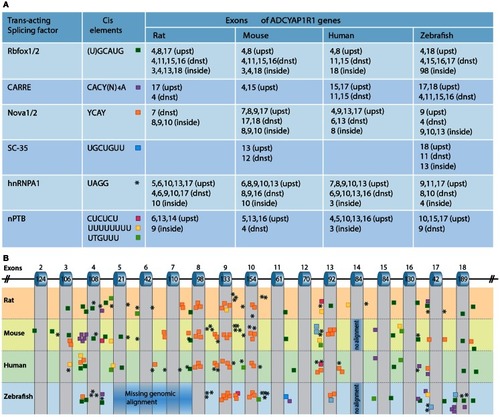
A scheme depicting the current knowledge on PAC1-mediated signaling cascades resulting in a variety of neuronal outcomes. PKA was shown to induce ERK1/2 thereby contributing to PACAP neuroprotective (Villalba et al., 1997; Vaudry et al., 2003; Falluel-Morel et al., 2006; Stumm et al., 2007) and neurotrophic (Ravni et al., 2006; Botia et al., 2007; Monaghan et al., 2008) functions. Action potential (AP) firing induces a CRCT1/CREB mediated neuroprotective effect, presumably through NMDA receptor activation and was shown to be initiated by PKA activation (Baxter et al., 2011). NMDA receptor was also shown to be indirectly activated by PACAP (Llansola et al., 2004) or cAMP/PKA signaling (Costa et al., 2009). PAC1/PKA signaling controls cellular apoptosis through inhibition of potassium channels (Mei et al., 2004; Castel et al., 2006; Pugh and Margiotta, 2006) or induction of calcium channels (Pugh and Margiotta, 2006). PAC1/PKA-mediated activation of ion channels leads to activity-dependent neuronal differentiation and synaptic plasticity (Lee et al., 1999; Maturana et al., 2002; Grumolato et al., 2003; Nishimoto et al., 2007). The tumor suppressor gene Lot1 known as a negative regulator of neuronal precursor proliferation was shown to be controlled by PAC1/cAMP/ERK signaling pathway (Fila et al., 2009). PAC1/PKA-dependent phosphorylation of Tau is involved in the control of granule cell migration during cerebellar development (Falluel-Morel et al., 2006). PAC1-mediated cAMP/ERK-dependent neurite outgrowth was shown to be regulated via a novel neuritogenic factor NCS (Emery and Eiden, 2012) or Epac/ERK (Gerdin and Eiden, 2007) pathways. PAC1/Epac was shown to regulate neuronal differentiation via activation of p38 kinase along with mobilization of Ca2+ from intracellular stores (Ster et al., 2007) as well as Epac/Rit-dependent pathway involving CREB signaling (Shi et al., 2006). PAC1 was demonstrated to induce Rit through TrkA/Shc/SOS signaling initiated by Src activation via dual Gs/Epac and Gi stimulation (Shi et al., 2010). PACAP also signals through Gq-linked PLC > IP3 > Ca2+/DAG > PKC, and PLD > phosphatidic acid (PA) pathways (Journot et al., 1994; Makhlouf and Murthy, 1997; Dejda et al., 2006). PAC1 is a mediator of gene transcription, neuronal differentiation, and synaptic development (Masmoudi-Kouki et al., 2006; Vaudry et al., 2007; Andero and Ressler, 2012). As an example of functional diversity caused by PAC1 alternative splicing two additional PAC1-hop1 signaling pathways are presented on the scheme. They depict PAC1-hop1/ARF dependent PLD activation (McCulloch et al., 2001) and internalization-dependent engagement of PI3Kγ/Akt activation (May et al., 2010). AC, adenylate cyclase; AGS, activator of G protein signaling; Akt, serine-threonine protein kinase PKB; cAMP, cyclic adenosine monophosphate; ARF, ADP(adenosine diphosphate) ribosylation factor; Caln, calcineurin; CaMK, calcium calmodulin kinase; CBP, creb binding protein; CRCT1, cysteine-rich C-terminal 1; CREB, cAMP responsive element-binding protein; DAG, diacyl glycerol; Epac, exchange factor directly activated by cAMP; ERK, extracellular signal-regulate kinase; G, guanine nucleotide-binding regulatory protein; IP3, inositol-1,4,5-triphosphate; JNK, c-Jun oncogene N-terminal kinase 1; Lot1, lost on transformation 1; NCS, neuritogenic cAMP sensor; NGF, nerve growth factor; NMDAR, N-methyl-d-aspartate receptor; p38, p38 mitogen-activated protein kinase; PA, phosphatidic acid; PI3K, phosphatidylinositol 3′ OH kinase; PKA, protein kinase A; PKC, protein kinase C;PLC, phospholipase; Raf, B-Raf proto-oncogene serine/threonine kinase;Ras, retrovirus-associated DNA sequences; Rap1, Rit, small GTPases of the RAS oncogene family; Sos, son of sevenless homolog 1; Src, sarcoma viral oncogen homolog; Tau, neuron-specific microtubule-associated protein; TrkA, tropomyosin-related kinase.
|