- Title
-
Naked1 Antagonizes Wnt Signaling by Preventing Nuclear Accumulation of β-Catenin
- Authors
- Van Raay, T.J., Fortino, N.J., Miller, B.W., Ma, H., Lau, G., Li, C., Franklin, J.L., Attisano, L., Solnica-Krezel, L., and Coffey, R.J.
- Source
- Full text @ PLoS One
|
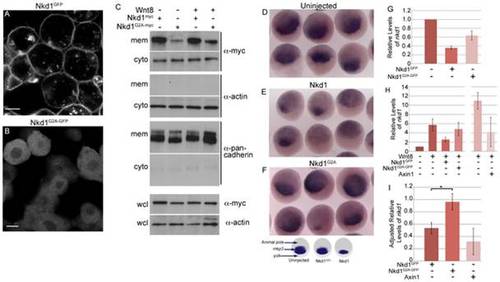
Plasma membrane localized Nkd1 is required for antagonizing Wnt signaling. nkd1GFP (A) or nkd1G2A-GFP (B) mRNA was injected into 1 of 4 blastomeres and allowed to develop until dome stage (4.3 hpf). Embryos were viewed live with confocal microscopy. (C) In a similar experiment, embryos injected at the one cell stage with either nkd1myc or nkd1G2A-myc in combination with wnt8, were collected at dome stage and fractionated into cytoplasmic (cyto) or plasma membrane (mem) fractions. Western blots of fractions were probed with anti-myc. This blot was also probed with anti-actin or anti-pancadherin to determine purity of fractions. Each lane represents the equivalent of 1 embryo from an average of 10 embryos. To determine total amount of protein, a portion of the pre-fractionated whole cell lysate (wcl) was western blotted and probed with anti-myc and anti-actin as a loading control. For the wcl, each lane represents the equivalent of 0.75 of an embryo from an average of 10 embryos. (D, E, F) Embryos were injected at the one cell stage with either nkd1 (E) or nkd1G2A (F) RNA and harvested, along with uninjected controls (D), at sphere stage (3.8 hpf). Embryos were processed for whole mount in situ hybridization using a mkp3 anti-sense probe [38]. The schematic below (F) summarizes the whole mount in situ hybridization data, identifies the different regions of the embryo and the domain of mkp3 expression in the dorsal organizer region. (G) Embryos were injected at the 1 cell stage with nkd1GFP or nkd1G2A-GFP, harvested at dome stage and total RNA was isolated and reversed transcribed. qRT-PCR was performed using endogenous nkd1 as readout. The 52 untranslated region of nkd1 was used as a target, the sequence of which was not incorporated into the expression vectors. Values are relative to uninjected embryos which was arbitrarily set to 1 (N = 3). (H) Quantification of endogenous nkd1 levels by qRT-PCR in the presence of Wnt8. (N = 6 for Wnt8; Wnt8+Nkd1GFP; Wnt8+Nkd1G2A-GFP). The Wnt8+Axin experiments (N = 3) were done independently of the others, which showed higher induction of nkd1 by Wnt8 alone, compared to the other experiments and relative to uninjected. (I) To adjust for the differences between experiments described in (H), the ratio of endogenous levels of nkd1 was determined for Wnt8+Nkd1GFP:Wnt8 (N = 6), Wnt8+Nkd1G2A-GFP:Wnt8 (N = 6) and Wnt8+Axin1:Wnt8 (N = 3) for each individual experiment and then averaged. (* = Students t-test, p = 0.0252). A level of one indicates no effect. Error bars represent standard error. Scale bar in A,B represents 10 μm. EXPRESSION / LABELING:
|
|
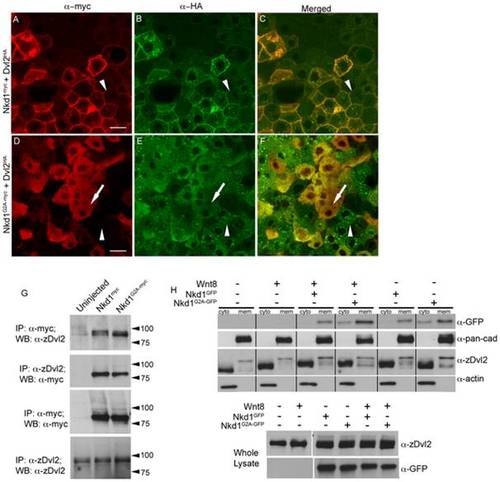
Nkd1 co-localizes with Dvl2. (A–F) Dvl2HA mRNA was injected at the one cell stage followed by injection of nkd1myc or nkd1G2A-myc RNA into 1 of 4 blastomeres at the 4-cell stage. Embryos were harvested at 50% epiboly (5.25 hpf) and processed for immunohistochemistry using anti-myc probes (red) or anti-HA probes (green). Arrowheads in A-F identify a cell that is positive for Dvl2HA but negative for Nkd1myc (A–C) or Nkd1G2A-myc (D–F). Arrow in D–F indentifies a cell that is positive for both Dvl2HA and Nkd1G2A-myc. (G–H) Nkd1 interacts with Dvl2. (G) Embryos were injected at the one cell stage with nkd1myc or nkd1G2A-myc and harvested at dome stage (4.3 hpf) for co-immunoprecipations. Lysates were incubated with anti-zDvl2 or anti-myc antibodies for immunoprecipitation. Immunoprecipitates were western blotted and probed with anti-zDvl2 or anti-myc antibodies (WB). Each lane represents the equivalent of 17.5 embryos. (H) nkd1GFP or nkd1G2A-GFP RNAs were co-injected with wnt8 RNA at the one cell stage. At 4.3 hpf, embryos were harvested and fractionated to isolate cytoplasmic (cyto) and plasma membrane (mem) fractions. Anti-actin and anti-pan-cadherin antibodies determined the purity of the cytoplasmic and plasma membrane fractions, respectively. A portion of the whole cell lysate was recovered prior to fractionation (lower panel) to determine loading controls. (Note: the high percentage gel precluded observing Dvl mobility shifts induced by Wnt8 in the whole cell lysate). The predicted MW of Nkd1myc is 61 kD, but the observed MW is closer to 75 kD. We speculate that this is likely due to post-translational modifications of Nkd1. Each lane represents the equivalent of 1 embryo, averaged from 10 embryos. Scale bar represents 20 μm. |
|
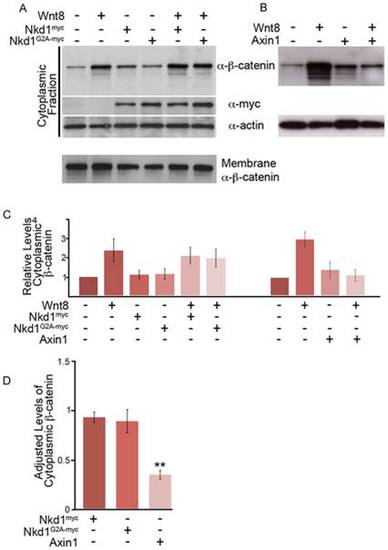
Nkd1 does not affect cytoplasmic levels of β-catenin. (A,B) Embryos were injected at the one stage with wnt8, nkd1myc or nkd1G2A-myc (A) or axin1 (B) and harvested at dome stage (4.3 hpf). Lysates were fractionated into plasma membrane (not shown) and cytosolic fractions, Western blotted and probed with anti-β-catenin, anti-myc and anti-actin antibodies. Each lane represents, on average, the equivalent of one embryo averaged from 10 embryos. (C) The average levels of cytosolic β-catenin from 6 independent experiments (Wnt8+/- Nkd1myc/Nkd1G2A-myc) or from 3 independent experiments (Wnt8+/-Axin1) were determined. Each lane was first normalized to actin levels and then compared to uninjected embryos, which was arbitrarily set to 1. D) To adjust for the differences between experiments described in (C), the ratio of cytoplasmic levels of β-catenin was determined for Wnt8+Nkd1myc:Wnt8 (N = 6), Wnt8+Nkd1G2A-myc:Wnt8 (N = 6) and Wnt8+Axin1:Wnt8 (N = 3) for each individual experiment and then averaged. (**-Students t-test, p = 0.0002). A level of one indicates no effect. Error bars represent standard error. |
|
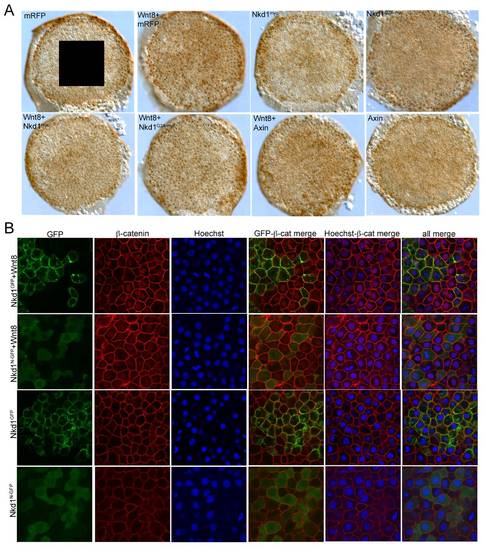
Nkd1 inhibits accumulation of nuclear β-catenin. (A–I) Embryos were injected at the one cell stage with mRFP (A,B); wnt8+mRFP (C); nkd1myc (D); nkd1G2A-myc (E); wnt8+nkd1myc (F); wnt8+nkd1G2A-myc (G); wnt8+axin1 (H); and axin1 alone (I). At dome stage (4.3 hpf) embryos were collected and processed for whole-mount immunohistochemistry with anti-β-catenin. The expression of nuclear β-catenin around the ventro-lateral margin of the embryo is due to endogenous Wnt8 activity. Note that Axin1 is also sufficient to prevent nuclear localization of β-catenin induced by endogenous Wnt8 activity. The box in (A) represents the size and position of analysis for (J). The rectangle in (A) depicts the regions shown in (B–I) which encompasses the ventro-lateral margins on either side of the embryo. (J) Blind counts of the number of cells expressing ectopic nuclear β-catenin was quantified (Table 2). Error bars represent standard error. To confirm the effect of Nkd1 on nuclear β-catenin, wnt8 RNA was co-injected with nkd1GFP (K) or nkd1N-GFP RNA (L) into 1 of 4 blastomeres, harvested at dome stage and processed for endogenous β-catenin staining. Regions of mosaic expression were chosen for analysis. Clones of Nkd1GFP or Nkd1G2A-GFP positive clones are outlined in white. Black and white images of GFP and β-catenin are shown for contrast. See also Fig. S1 for the complete set of images. (M-N) Quantification of the differences in nuclear β-catenin from six cells (Wnt8+Nkd1GFP) or 10 cells (Wnt8+Nkd1N-GFP) cells each for GFP positive/Hoechst positive and juxtaposed GFP negative/Hoechst positive cells. The graphs in (M) represents the two cells identified by asterisks in the right hand-most image of (K). The dashed lines delineate the nuclear grey levels. (N) The level of nuclear β-catenin was adjusted using the Hoechst staining. Only the grey values located between the dashed lines were evaluated. (p value = 0.0002). Error bars represent std error. Scale bar represents 20 μM. |
|
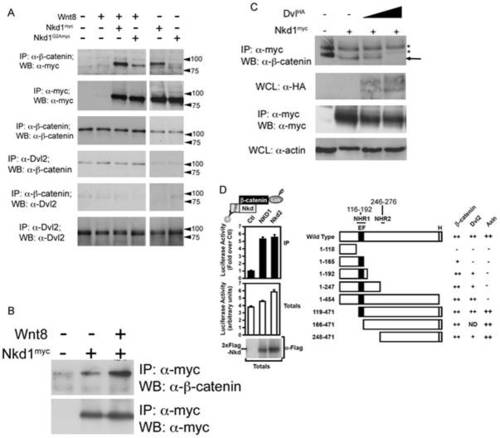
Nkd1 interacts with β-catenin. To determine if Nkd1 and β-catenin interact, wnt8 +/- nkd1myc or nkd1G2A-myc was injected at the 1 cell stage and harvested at dome stage for immunoprecipitation with anti-β-catenin (A). Each lane represents the equivalent of 17.5 embryos. Note, this experiment was part of a larger experiment involving the co-immunoprecipitations shown in Fig. 2 (B) and as such share some of the same controls. (B) nkd1myc +/- wnt8 was injected into 1 cell stage embyros and harvested at dome stage, immunoprecipitated with anti-myc and western blotted with anti-β-catenin. The interaction between Nkd1 and β-catenin is much stronger in the presence of exongenous Wnt. Slight non-specific binding of the anti-myc antibody is observed in the control lane. A no primary antibody control showed no banding pattern (not shown). C) nkd1myc was co-injected with increasing doses of dshHA (0pg, 125pg and 250pg) harvested at dome stage, immunoprecipitated with anti-myc and western blotted with anti-β-catenin. The astericks identify non-specific banding patterns. D) The interaction of luciferase-tagged β-catenin (β-catenin-Lux) with wild type Nkd1 and Nkd2 (left panel) and Nkd1 mutants (right panel) was tested using the LUMIER assay in HEK293T cells. Numbers above Wild Type Nkd1 refer to amino acid positions in human Nkd1. Axin data has been previously reported [28] and is shown here for completeness. Data are shown as the mean of 2 samples +/- standard deviation (left panel) and interaction intensities of less than 50% (-), 50–75% (+) and >75% (++) of wild type are indicated (right panel). clarity we refer to NHR1 as Naked Homology Region 1 (116–192aa), which encompasses the EF hand (143–154aa). The NHR1 region has also been referred to as NH2 [56] as well as EFX [10]. |
|
Nkd1 inhibits nuclear β-catenin. (A) Whole embryo analysis of nuclear β-catenin staining as per Fig. 4.) (B) Immunohistochemistry of Nkd1GFP and Nkd1N-GFP co-injections with wnt8. Injections are labeled along the left side, staining is identified along the top. |