- Title
-
Controls of meiotic signaling by membrane or nuclear progestin receptor in zebrafish follicle-enclosed oocytes
- Authors
- Hanna, R.N., and Zhu, Y.
- Source
- Full text @ Mol. Cell. Endocrinol.
|
Expressions of membrane progestin receptor α (mPRα), mPRβ and nuclear progestin receptor (nPR or Pgr) in zebrafish oocytes and transfected human embryonic kidney 293 cell line (HEK293). (A) Representative Western analyses of mPRα, mPRβ and nPR proteins in follicle-enclosed stage IV oocytes (>650 μm) sampled at 13:00 (after spawning), 21:00 (evening), or 6:00 h (before spawning). The same analysis was also repeated in denuded oocytes and detached follicular cell layers collected at 6:00 (right panel in A). (B) Changes of mPRα, mPRβ, and nPR transcripts in detached follicular layers and denuded stage IV oocytes (n = 8) analyzed by real-time quantitative PCR (qRT-PCR). Different letters above the error bars indicate that those groups are significantly different from each other at P < 0.05. UD: under detection limit. (C) Localization of zebrafish mPRα-EGFP, mPRβ-EGFP, and nPR-EGFP proteins in transfected HEK293 cells. White arrows indicate plasma membrane localization; black arrows indicate intracellular compartment localization; and asterisks indicate cytoplasmic/nuclear localization. Each experiment was repeated at least three times. |
|
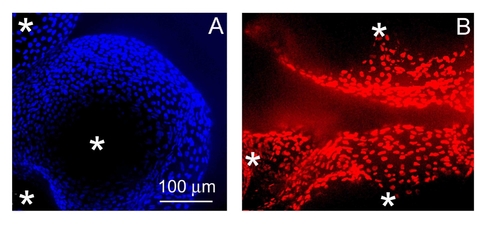
Representative surface images of follicle-enclosed stage IV oocytes (A–F) compared to denuded stage IV oocytes (G–L), photographed using a scanning electronic microscope (SEM) or a confocal scanning microscope. (A), (B), (G) and (H) are recorded under the SEM; (B) and (H) show the magnified surface of the same follicle-enclosed oocyte in picture A or the denuded oocyte in picture G. Images of (C–F) and (I–L) were photographed under a confocal microscope. (C), (E), (I) and (K) are projected confocal images of follicle-enclosed oocytes (C and E) compared to denuded oocytes (I and K) stained with 4′,6-diamidino-2-phenylindol (DAPI, C and I) or propidium iodide (PI, E and K). (D), (F), (J) and (L) were taken using differential interference contrast (DIC), corresponding to the same oocytes in the pictures directly above (C), (E), (I) and (K), respectively. |
|
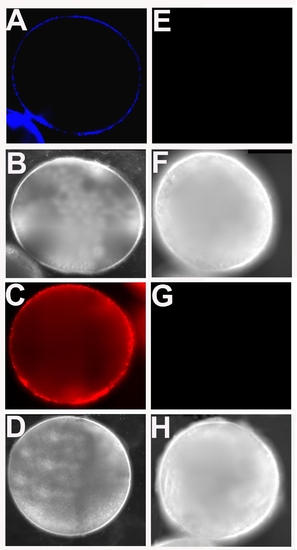
(A) Expression of mPRα-GFP fusion proteins in a representative oocyte microinjected with the transgenic transcript. Follicular cells were removed for better imaging under a fluorescent microscope; (B) Western analyses of expressions of transgenic proteins for membrane progestin receptor α (mPRα), mPRβ, or nuclear progestin receptor (nPR) in protein extractions of purified plasma membrane (for mPRs) or cytosolic fraction (for nPR) from stage IV zebrafish oocytes. These oocytes were microinjected ex vivo with fused transcripts generated by fusing mPRα, mPRβ, or nPR in front of enhanced green fluorescent protein. Samples were collected following 5-h incubation in a culture medium. Representative results are shown as positive reactions (bands) to an anti-GFP antibody Con: control oocytes injected with a GFP carrier plasmid. |
|
Supplemental Fig.1 Hanna et al |
|
Supplemental Fig. 2. Hanna et al Stage III Follicle-Enclosed Oocytes or Denuded Oocytes |
|
Supplemental Fig. 3. Hanna et al |
|
Supplemental Fig. 4. Hanna et al |
|
Supplemental Fig. 5. Hanna et al |
|
Supplemental Fig. 6. (A) Hanna et al |
Reprinted from Molecular and Cellular Endocrinology, 337(1-2), Hanna, R.N., and Zhu, Y., Controls of meiotic signaling by membrane or nuclear progestin receptor in zebrafish follicle-enclosed oocytes, 80-8, Copyright (2011) with permission from Elsevier. Full text @ Mol. Cell. Endocrinol.