- Title
-
An Enzymatic Mechanism for Generating the Precursor of Endogenous 13-cis Retinoic Acid in the Brain
- Authors
- Takahashi, Y., Moiseyev, G., Chen, Y., Farjo, K., Nikolaeva, O., and Ma, J.X.
- Source
- Full text @ FEBS J.
|
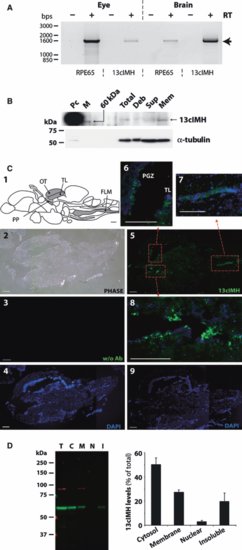
Localization of zebrafish 13cIMH in the brain and eye. (A) RT-PCR analysis of RPE65 and 13cIMH using RNA from the zebrafish eye and brain. RT-PCR was performed in the absence (-) and presence (+) of RT to exclude possible genomic DNA contamination. The arrow indicates the expected product size of 1.6 kb. (B) Western blot analysis of endogenous 13cIMH in the total membrane fraction of the brain. Cellular proteins (2.5 μg) of 293A-LRAT cells expressing 13cIMH were used as a positive control (Pc). Equal amounts (50 μg) of total zebrafish brain homogenates (Total), unbroken cell debris (Deb), supernatants following centrifugation (Sup) and total membrane fraction (Mem) were resolved by 8% SDS/PAGE and transferred onto the membrane. The endogenous 13cIMH expression was confirmed by western blot analysis (upper panel), and then the membrane was stripped and reblotted with an antibody for tublin (Abcam; lower panel). (C) Immunohisotochemistry of 13cIMH in the zebrafish brain. (C1) The diagram shows a drawing sagittal section of zebrafish brain (modified from Rupp et al. [36]). Gray-colored regions indicate the stained areas by immunohistochemistry. PP, periventricular pretectum; FLM, fasciculus longitudinalis medialis. (C2) A phase contrast image of a sagittal section of zebrafish brain. (C3, 4) The brain section was incubated without the primary antibody for 13cIMH (C3; FITC channel, c4; DAPI). (C5–9) The brain section was incubated with the primary antibody for 13cIMH. Green fluorescence indicated the signals of 13cIMH at low magnification (C5; 13cIMH and c9; DAPI) and at high magnification from the boxed areas in c5: torus longitudinalis (TL) (C6–8). Scale bar = 200 μm. (D) Subcellular localization of 13cIMH in cultured cells. Forty-eight hours post-transfection of the 13cIMH plasmid, the cells were harvested and separated into four subcellular fractions by the FractionPrep™ kit (BioVision, Mountain View, CA, USA). Equal amounts of fractionated proteins (25 μg for total protein, 5 μg each fraction) were employed for western blot analyses using anti-13cIMH serum. T, total cell lysates; C, cytosolic; M, membrane; N, nuclear fractions; I, detergent-insoluble fraction. The level of 13cIMH in each fraction was quantified by densitometry and expressed as the percentage of total 13cIMH (mean ± SEM) from four independent experiments. EXPRESSION / LABELING:
|
|
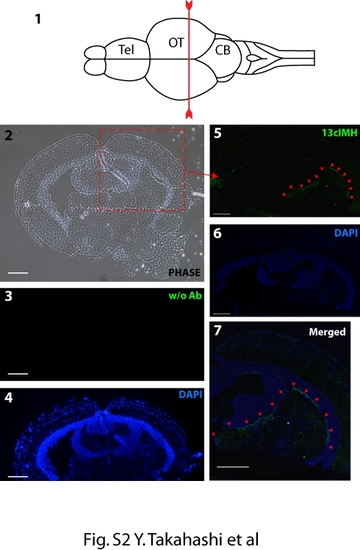
Immunohistochemistry of the cross section at the optic tectum of zebrafish brain. (1) The diagram shows zebrafish brain (modified from Castro, A. et al. [3]) and a line indicates the cutting line. Tel; Telencephalon, OT; Optic Tectum CB; Cerebellum. (2) A phase contrast image of a cross section of zebrafish brain. (3, 4) The brain section was incubated without the monoclonal antibody for 13cIMH (Negative control, 3; FITC channel, 4; DAPI). (5-7) The brain section was incubated with the monoclonal antibody for 13cIMH. Green fluorescence signals, indicated by red arrows, for 13cIMH (5; 13cIMH, 6; DAPI, 7; merged) were detected in the preventricular grey zone (PGZ) of optic tectum (OT). Scale bar = 200 μm. EXPRESSION / LABELING:
|
|
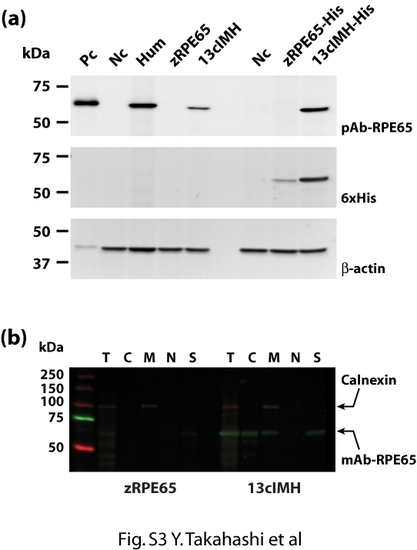
Specificity of the anti-RPE65 antibodies to recombinant zebrafish 13cIMH. (a) The expression plasmids were transfected into 293A cells and cultured for 48 hours. The cells were harvested, equal amounts (20 μg) of total cellular protein were resolved by SDS-PAGE, and the protein expression was confirmed by Western blot analysis with a polyclonal anti-human RPE65 [4], mouse monoclonal anti-His-tag, and goat polyclonal anti-β-actin antibodies at same time. Pc, positive control (bovine RPE microsomal protein); Nc negative control (cells expressing RFP); Hum, human RPE65; zRPE65, zebrafish RPE65 without 6xHis-tag; 13cIMH, zebrafish 13cIMH without 6xHis-tag; zRPE65-His, zebrafish RPE65 with 6xHis-tag; 13cIMH-His, zebrafish 13cIMH with 6xHis-tag. Subcellular fractionation was performed as explained in Materials and Methods. The polyclonal antibody recognized zebrafish 13cIMH but not zebrafish RPE65. (b) The cells expressing 13cIMH were fractionated. The same amounts of protein from each fraction (5 μg) were blotted with a monoclonal anti-human RPE65 antibody (Millipore, Billerica, MA) and a rabbit polyclonal antibody to calnexin (ER membrane marker, Abcam, Cambridge, MA). The monoclonal antibody recognized zebrafish 13cIMH but not zebrafish RPE65 (zRPE65). T; total cell lysates, C; cytosolic, M; membrane, N; nuclear fractions and S; detergent-insoluble fraction including cytoskeleton and inclusion body. |