- Title
-
Amyloid precursor protein is required for convergent-extension movements during Zebrafish development
- Authors
- Joshi, P., Liang, J.O., Dimonte, K., Sullivan, J., and Pimplikar, S.W.
- Source
- Full text @ Dev. Biol.
|
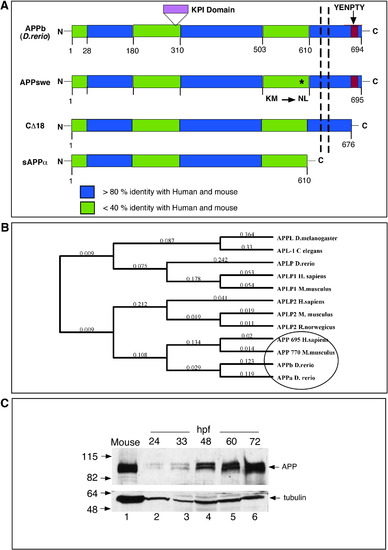
Homology and phylogenetic analysis of APP. (A) Schematic representation of zebrafish APPb and the truncation mutants of human APP used in this study. Zebrafish APPb is a 694-residue-long protein with the extracellular N-terminal domain, a transmembrane domain (shown by the vertical dashed lines), and an intracellular C-terminal domain that contains the EYNPTY motif (red box). APP family proteins show 3 highly conserved domains (blue regions) that are more than 80% identical among each other, and are separated by less conserved regions (green regions) that are less than 40% identical at the protein level. APPa contains a 40-residue-long KPI domain (shown in purple), which is not present in APPb. The location of the Swedish mutation in APP (where residues ‘KM’ are mutated to ‘NL’) is shown by an ∗. APP-CΔ18 lacks the last 18 C-terminal residues, including the EYNPTY motif. Soluble sAPPα, which is truncated at the α-cleavage site and lacks both the membrane-spanning domain and the intracellular domain. The percentage homology is obtained by using Blast at ncbi.nlm.nih.gov or ClustalW in MacVector 7.2.2, both using Blosum matrices. (B) Phylogenetic groupings between APP family proteins from different species. The tree was generated by using default settings following multiple alignment using ClustalW and Neighbor-joining algorithms. Zebrafish APPa and APPb group closer with mouse and human APP compared to invertebrate APP proteins. (C) Zebrafish APP levels increase during embryogenesis. Western blot analysis of zebrafish proteins at different stages of development. Deyolked embryos were collected at the indicated hpf, resolved by SDS-PAGE, and probed with anti-C-terminal antibody that was raised to the last 15 residues of human APP. Total protein homogenate from a mouse brain was loaded in lane 1 as a positive control. Like mouse APP, zebrafish APP migrates as a closely spaced doublet at ∼ 100–110 kDa. The lower part of the nitrocellulose membrane was probed with anti-α-tubulin antibody (DM1A). |
|
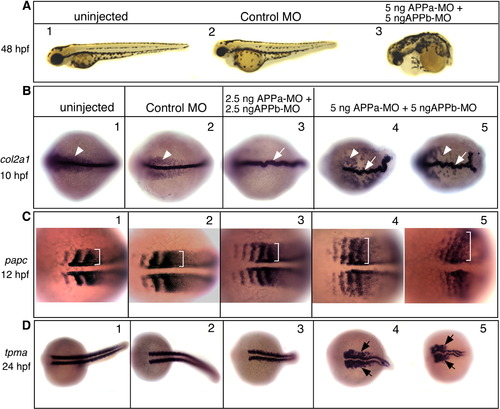
Knockdown of APPa and APPb results in convergent-extension defects. (A) Lateral views, anterior to the left and dorsal up, of live embryos visualized at 48 hpf. Embryos were uninjected (1) or injected with control morpholino (2), or injected with a mixture of 5 ng each of APPa-MO and APPb-MO (3). Compared to the uninjected or control-MO-injected embryos, embryos injected with APPa and APPb morpholinos have dramatic phenotypic changes that include a short body axis, a short curly tail, and small eyes. (B–D) Zebrafish APP function is required for normal convergent-extension movements. Embryos were uninjected (each panel 1), injected with control-MO (each panel 2), or injected with a low dose (each panel 3) or a high dose (each panel 4, 5) of APPa and APPb-MO mixture. (B) Embryos were fixed at the tail-bud stage (10 hpf) and labeled with type II Collagen 1-α (col2a1) RNA probe. Uninjected or control-MO-injected embryos have a thin, long, and straight notochord (arrows). The notochord is slightly thicker and misshaped in the low dose MO embryos (3), and dramatically crooked in the high dose embryos (4, 5). At the higher MO dose, endodermal precursor cells (arrowheads) are found further away from the notochord. (C) Embryos were fixed at the 4–6 somite stage (12 hpf) and labeled with paraxial protocadherin (papc) RNA probe. Brackets in each panel show somite width. Knock down of APP function results in wider somites in a dose-dependent manner. (D) Embryos were fixed at 24 hpf and labeled with α-tropomyosin (tpma) RNA probe. Compared to the control embryos, the MO-injected embryos have a short tail (3) and severely deformed and widened myotomes, especially at the higher MO dose. |
|
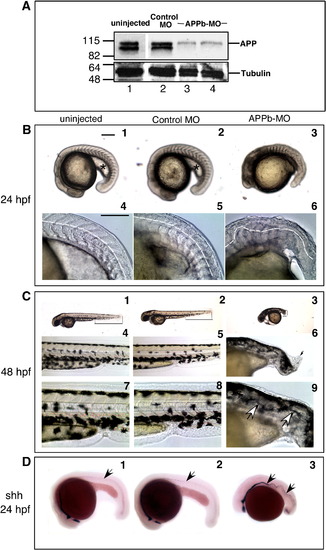
Knock down of APPb protein alone causes aberrant convergent-extension movements. (A) Knock down of APP levels by APPb-MO. Embryos were uninjected (lane 1) or injected with control-MO (lane 2) or with 5 ng (lane 3) or 10 ng (lane 4) of APPb-MO at the 1–2 cell stage, and harvested 48 hpf. Total protein extract was prepared from deyolked embryos and analyzed by SDS-PAGE and Western blot using anti C-terminal antibody. There is a dramatic reduction in APP levels (top panel) in APPb-MO-injected embryos (lanes 3, 4) compared to the controls. The same membrane was stripped and reprobed with anti-tubulin antibody (bottom panel). (B) Aberrant development in APPb knock down embryos. Morphology of live embryos at 24 hpf. Lateral views, anterior to the left and dorsal to the top. Embryos were uninjected (1), or injected with control-MO (2), or APPb-MO at 10 ng (3). Compared to uninjected or control-MO-injected embryos, APPb-MO-injected embryos had a shorter body axis and a deformed tail. At high magnification, the tail region of APPb-MO-injected embryos (6) show undulating curvature (highlighted by dotted lines) and deformed somites (4, 5). Scale bars are represented on the top right on the left panel, and equal 0.5 mm. (C) The curly tail defect is more pronounced at 48 hpf. Lateral views, anterior to the left and dorsal to the top, of live embryos at 48 hpf. Compared with uninjected (1) or control-MO-injected embryos (2), APPb-MO-injected embryos exhibit severely reduced body length and a short, curly tail (3; shown with thin black lines from hind yolk to the tail tip in each embryo). At high magnification, a mass of disorganized cells could be visualized at the tail tip (black arrow in 6) These abnormal growths were sometimes seen in the APPb-MO-injected embryos, but never in control embryos. An even higher magnification of the notochordal region of the tail reveals severe undulations in APPb-MO-injected embryos (shown by arrows in 9), not present in uninjected (7) and control-MO-injected embryos (8). (D) Aberrant notochord development in APPb knock down embryos. Embryos were fixed 24 hpf and labeled with an RNA probe for sonic hedgehog (shh), a marker for notochordal and floor plate cells. Arrows show severely undulating notochord in APPb-MO-injected embryos (3) compared to control embryos (1, 2). |
|
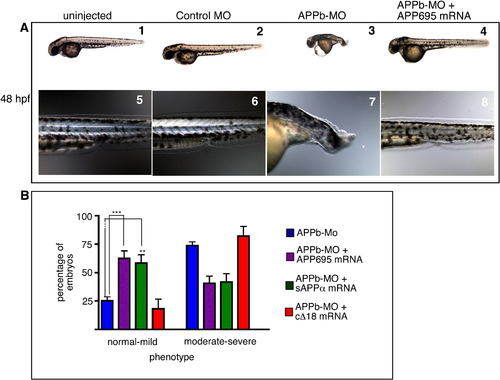
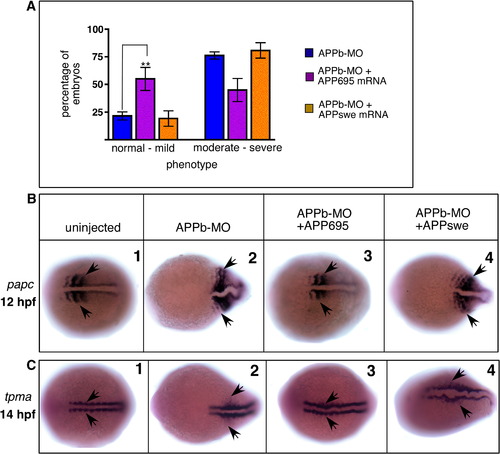
Ectopic expression of human APP695 rescues the APPb morphant phenotype.( A) Morphology of live embryos at 48 hpf. Lateral views, anterior to the left and dorsal up. Embryos were uninjected (1), control-MO-injected (2), APPb-MO-injected (3), or co-injected with APPb-MO and APP695 mRNA (4). Note that co-injection with APP695 mRNA rescued the developmental defects. A higher magnification of the tail region showed an elongated ‘stack of pennies’ notochord in uninjected (5) and control-MO-injected (6) embryos, compared to an undulating notochord and a curly tail in APPb-MO-injected embryos (7). Embryos co-injected with APPb-MO and APP695 mRNA (8) revert to an elongated tail and ‘stack of pennies’ notochord that is similar to controls. (B) Quantification of embryo rescue through co-injection of mRNA. Injection of 10 ng of APPb-MO causes the defective phenotype (shortened axis, curly tail) in 75% of embryos (blue bars). Co-injection with mRNA encoding full-length APP695 (purple bars) results in an increase in the normal-to-mild phenotypes, compared to APPb-MO-injected embryos (blue bars) that have increased moderate-to-severe phenotypes, indicating rescue of the curly tail morphant phenotype with APP695 mRNA. ∗∗∗ is p = 0.0002, p < 0.05 in two-tailed unpaired t-test; Gaussian distribution; N of experiments = 14; N of embryos ∼ 300. Similarly, embryos co-injected with APPb-MO with sAPPα ectodomain (green bars) showed an increase in normal-to-mild phenotypes in comparison to APPb-MO-injected embryos. ∗∗p = 0.0037 for sAPPα with Mann–Whitney test N of experiments = 8. Total N of embryos analyzed = 143. In contrast, cΔ18 mRNA co-injected embryos (red bars) had a similar number of normal-to-mild phenotypes as APPb-MO-injected embryos, indicating cΔ18 mRNA does not rescue the morphant phenotype. p > 0.05, Mann–Whitney test for cΔ18. Error bars represent ± standard error of mean (S.E.M). PHENOTYPE:
|
|
APPswe does not rescue APPb morphant phenotype. (A) Quantification of embryos showing rescue from the short, curly tail phenotype. Whereas approximately 25% of embryos injected with APPb-MO alone appeared normal (blue bars), co-injection with wild-type APP695 mRNA (purple bars) showed over 50% embryos to be normal. There was a concomitant decrease in the number of embryos showing the severe phenotype. However, when injected with the same amount of mRNA encoding APPswe, there was no increase in embryos with normal phenotype, nor was there any decrease in embryos showing the severe phenotype (yellow bars). p > 0.05 by ANOVA. Error bars are ± S.E.M. ∗∗ p < 0.0044 between APPb-MO-injected embryos and those co-injected with APP695 mRNA. (B, C) APPswe mRNA does not rescue the defective convergent-extension phenotype. Embryos were fixed at 12 hpf and labeled with papc RNA probe (B) or fixed at 14 hpf and labeled with tpma RNA probe (C). In situ hybridization with papc at the 4–6-somite stage reveals wider somites in APPb-MO-injected embryos (2), which appear normal in embryos co-injected with APP695 mRNA (3) but not with APPswe mRNA (4). Similarly, in situ hybridization with tpma at the 6–8 somite stage show wider myotomal precursors and midline in APPb-MO-injected embryos (2) compared to uninjected embryos (1). Co-injection with APP695 mRNA (3) but not APPswe (4) reverses the increased width of the myotomal precursors and midline to near the width in uninjected embryos. EXPRESSION / LABELING:
PHENOTYPE:
|
|
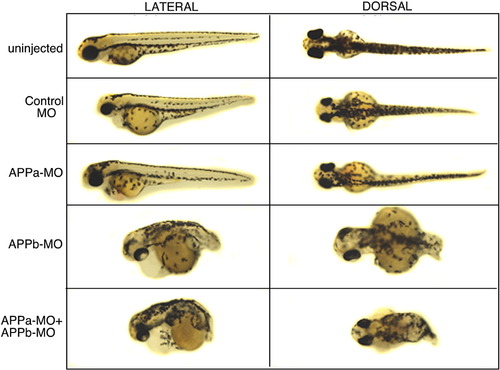
Morphological defects observed at 48 hpf in embryos injected with APP morpholinos. Left panel shows lateral views of embryos with anterior view to the left and posterior to the right. Right panel shows dorsal views. From top to bottom: uninjected (first row) and control-MO-injected (second row) embryos showed an elongated body axis and tail. APPa morpholino alone (APPa-MO) (third row) showed whole embryo morphology very similar to uninjected and control-MO-injected embryos (first and second rows). By contrast, APPb morpholino (APPb-MO) (fourth row) alone showed a drastically shortened body axis and a severe curly tail. APPa and APPb morpholino injected together (APPa + APPb-MO) (bottom row) also showed a drastically reduced body axis and a severe curly tail, similar to APPb-MO alone. |
|
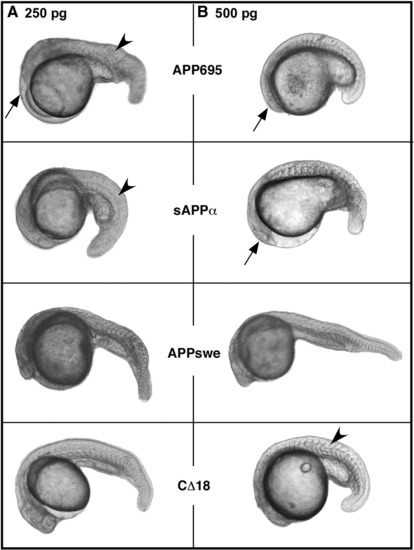
Effects of overexpression of APP695 and its mutant constructs on embryonic development. Zebrafish embryos were injected with mRNA from APP695 or indicated mutant constructs at 1–2 cell stage and observed at approximately 36 hpf. Embryos in column A were injected with 250 pg dosage and in column B with 500 pg dosage of mRNA encoding various APP constructs. Representative images are shown. Overexpression of each construct results in embryos with posteriorized phenotype with smaller head, smaller eyes (arrows) and thicker trunks (arrowheads). At either dose of mRNA, APP695 is most effective in inducing the phenotype whereas CΔ18 is least effective. None of the constructs induce phenotypic changes below 200 pg dosage while most constructs, except CD18, cause severe phenotypic changes at 500 pg and above. APP695 injected embryo showing posteriorization are shown at the top while representative images of embryos injected with indicated dose of various mutant constructs are shown below. |
|
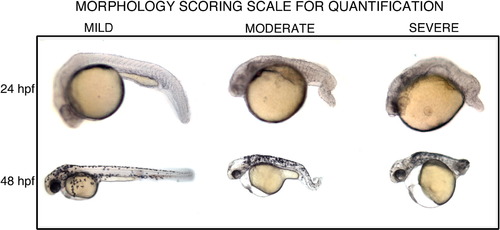
Representative images of embryos showing mild, moderate or severe morphological defects. An arbitrary scale for scoring the severity of the phenotypic defect (short embryo axis, curly tail) in the embryos at 24 and 48 hpf. Representative images of embryos scored as normal or mild (left), moderate defect (middle), or severe phenotype (right). Embryos were scored with the experimenter blind to the treatment of each embryo. |
|
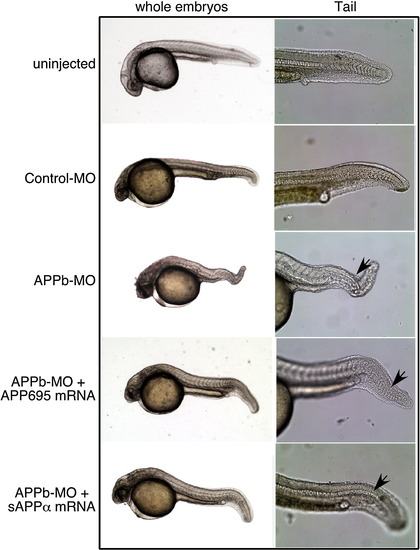
Rescue with sAPPα. Left column: whole embryo morphology; right column: tail region magnified. Top and second rows: uninjected and control-MO-injected embryos. Third row, APPb-MO-injected embryo with a curly tail. Fourth row, embryo co-injected with APPb-MO and APP695 mRNA with a tail similar to the control-MO embryo, but with a slight curvature, and disorganized tail fin. Fifth row, embryo co-injected with APPb-MO and sAPPα mRNA with a tail similar to control-MO, but again with a slight curvature and tail fin not fully formed. |
|
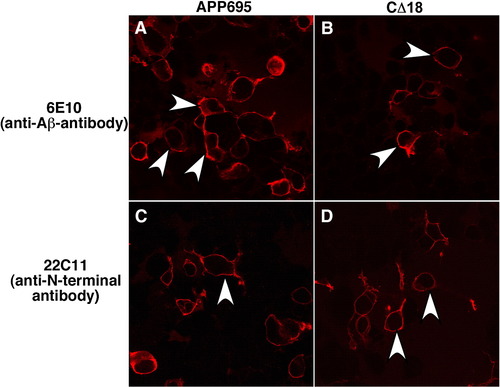
APP695 and APP-CΔ18 are present at the plasma membrane in HEK 293 cells. Confocal images of HEK 293 cells transiently transfected with pCS2 + APP695 or the deletion mutant pCS2 + APP-CΔ18. The cells were fixed in paraformaldehyde and probed with 6E10 antibody against the Aβ region or with 22C11 antibody against the extracellular region of APP, followed by an anti-mouse secondary conjugated with Alexa 594. The immunofluorescence images show that full-length APP695 (A) or CΔ18 truncation protein (B) can be detected at the plasma membrane when detected with 6E10 antibody. The immunofluorescence pattern is indistinguishable between APP695 and CD18 (white arrowheads). 22C11 antibody that recognizes the N-terminal domain of APP gave a similar staining pattern (C, D). |
Reprinted from Developmental Biology, 335(1), Joshi, P., Liang, J.O., Dimonte, K., Sullivan, J., and Pimplikar, S.W., Amyloid precursor protein is required for convergent-extension movements during Zebrafish development, 1-11, Copyright (2009) with permission from Elsevier. Full text @ Dev. Biol.