- Title
-
FGF signaling is required for {beta}-catenin-mediated induction of the zebrafish organizer
- Authors
- Maegawa, S., Varga, M., and Weinberg, E.S.
- Source
- Full text @ Development
|
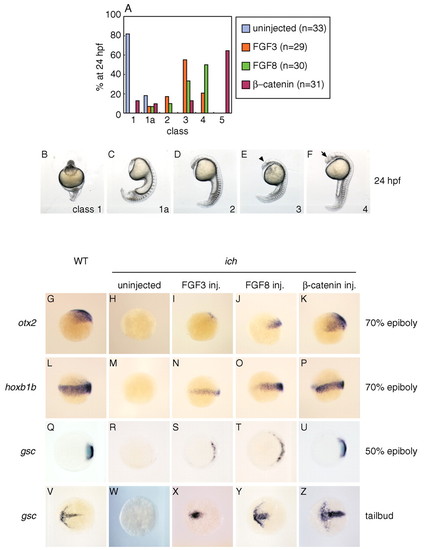
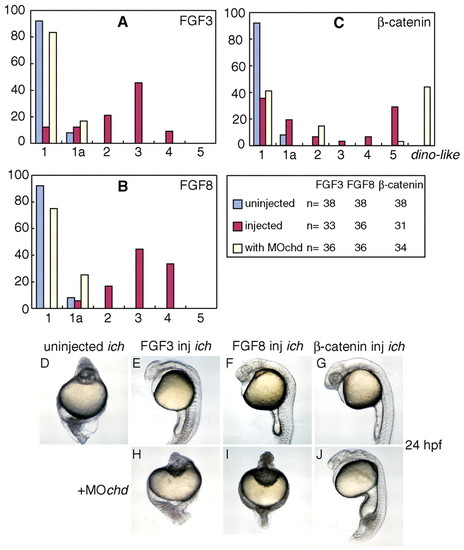
FGF3 and FGF8 can partially restore AP and DV patterning in ichabod embryos. (A) Partial rescue of ichabod embryos injected with RNAs for FGF3 (orange) or FGF8 (green), compared with uninjected ichabod embryos (blue) or β-catenin-injected embryos (red). (B-F) Phenotypic classes of 24 hpf ichabod embryos (Kelly et al., 2000; Tsang et al., 2004) used for the classification in A and in subsequent figures. Class 1 embryos lack head and trunk; class 1a lack all structures anterior to spinal cord; class 2 lack structures anterior to hindbrain; class 3 form deficient forebrain; class 4 lack notochord (as do the previously mentioned classes) but have a complete AP axis. Only class 5 embryos (not shown) appear normal at 24 hpf. The arrowhead in E indicates the position of the midbrain/hindbrain boundary; the arrow in F points to aneye. (G-Z) FGFs restore expression of markers of anterior and posterior neurectoderm and organizer. FGF3 and FGF8 induce otx2 (H-J), hoxb1b (M-O) and gsc (R-T,W-Y). Expression is compared to wild-type (G,L,Q,V), uninjected ichabod (H,M,R,W) and ichabod embryos injected with β-catenin RNA (K,P,U,Z). Embryos are shown at 70% epiboly (G-P, lateral views), 50% epiboly (Q-U, animal pole views) and 10 hpf tailbud stage (V-Z, dorsal views). |
|
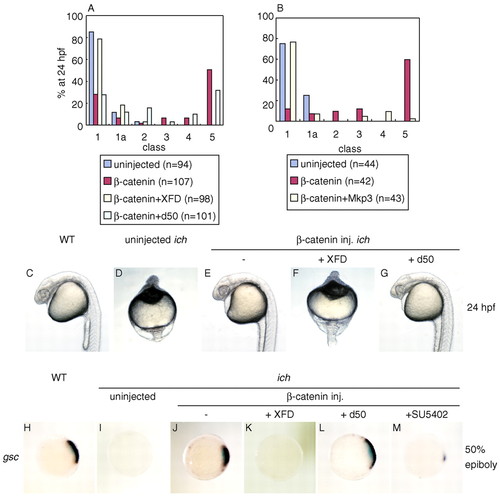
FGF signaling is required for β-catenin-induced organizer formation. (A,B) Distribution of phenotypes of ichabod embryos injected with RNAs for β-catenin alone (red) or co-injected with RNAs for β-catenin and the inhibitor of FGF signaling (yellow) XFD (A), MKP3 (B) or the inactive FGF receptor, d50 (light blue, A). Uninjected ichabod embryos were severely ventralized (dark blue). β-catenin can partially or completely rescue most embryos, but both the dominant negative FGF receptor, XFD (A) or MKP3(B) inhibit this rescue. The non-functional FGF receptor, d50, had no effect on β-catenin rescue (A). (C-G) Typical representative embryos are shown for each condition of treatment. (H-M) FGF signaling is essential for formation of the organizer, as assayed by gsc expression. At 50% epiboly, wild-type embryos robustly express this gene in the shield region (H), but ichabod embryos are devoid of expression (I). β-catenin induction of gsc in ichabod embryos (J) is abolished by coexpression of XFD (K) or by injection of SU5402 (M) but not d50(L). Embryos in H-M are shown in animal pole views. EXPRESSION / LABELING:
|
|
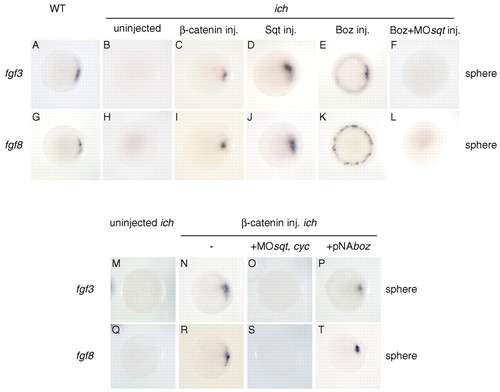
FGF3 and FGF8 act downstream of Squint and Bozozok. Sqt (D,J), Boz (E,K) and β-catenin (C,I) can induce expression of fgf3 (B-E) and fgf8 (H-K). fgf3 and fgf8 are expressed in the future dorsal region in wild-type (A,G) but not in uninjected ichabod (B,H) embryos. Boz induces fgf3 and fgf8 through Sqt (E,F,K,L). In ichabod embryos, Boz can induce weak expression of fgf3 (E) and fgf8 (K), but this expression is abolished in the presence of MOsqt (F,L). (M-T) Nodal proteins, but not Boz, are required for β-catenin induction of fgf3 and fgf8. The induction of fgf3 and fgf8 observed in ichabod embryos injected with β-catenin RNA (compare M with N and Q with R) is abolished in the presence of MOs against sqt and cyc (O,S) but not by pNAboz (P,T). Embryos are shown at sphere stage. EXPRESSION / LABELING:
|
|
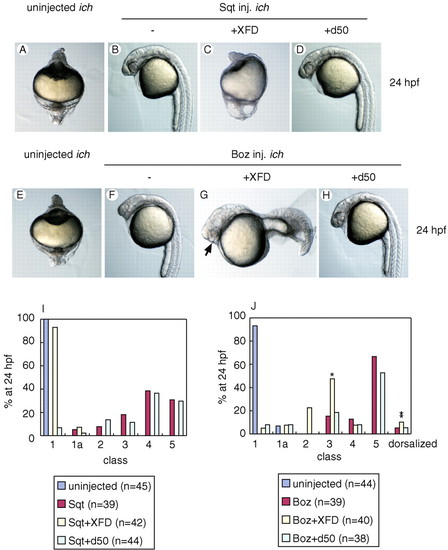
FGF signaling is essential for rescue of ichabod by Squint but not by Bozozok. XFD completely blocks rescue by Sqt (A-D) but not Boz (E-H). Typical examples are shown of uninjected ichabod embryos (A,E), and ichabod embryos injected with RNAs for sqt (B), boz (F), sqt and XFD (C), boz and XFD (G), sqt and d50 (D) or boz and d50 (H). Many embryos injected with boz and XFD RNAs form eyes (arrow, G). (I,J) Phenotypic classification of the embryos shown in A-D(I) or E-H (J). The asterisk indicates embryos similar to those shown in G and the double asterisk class is highly dorsalized. Embryos are shown at 24 hpf. |
|
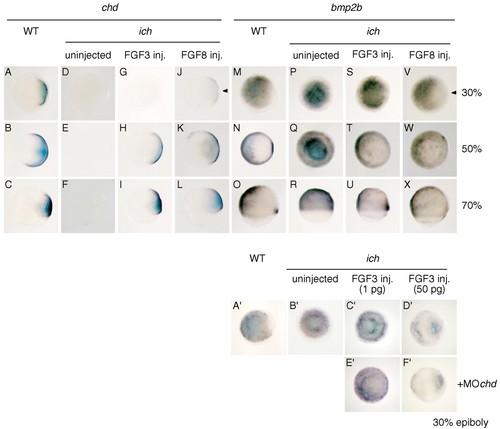
Coordinate induction of chd and repression of bmp2b by FGFs. (A-X) ichabod embryos injected with 1 pg FGF3 (G-I,S-U) or 0.1 pg FGF8 (J-L,V-X) were compared with uninjected ichabod (D-F,P-R) and wild-type embryos (A-C,M-O) for chd (A-L) or bmp2b (M-X) expression. Thirty percent epiboly and 50% epiboly embryos are shown in animal pole views; 70% epiboly embryos are shown in lateral views, with dorsal to the right in those embryos with a recognizable dorsal side. In every case of chd induction there was also repression of bmp2b [induction and repression of these genes in 30% epiboly embryos after injection with FGF8 RNA (J,V) is indicated with arrowheads]. Thirty percent epiboly embryos after injection with FGF3 RNA, the one condition that did not result in chd induction (compare D,G), also did not show bmp2b repression (compare P,S). (A'-E') ichabod embryos injected with 1 pg (C',E') or 50 pg (D`F') of FGF3 RNA were compared with uninjected ichabod (B') or wild-type (A') embryos for bmp2b expression at 30% epiboly (shown in animal pole views). The lower amount of FGF RNA did not lead to repression of bmp2b but the higher amount of FGF3 RNA did repress the gene. Co-injection of MOchd (E',F') did not affect the level of bmp2b expression at either RNA input (compare C',E' or D`F'). EXPRESSION / LABELING:
|
|
chordin is required for FGF-induced dorsal axis formation in ichabod embryos. Phenotypic distributions for ichabod embryos injected with FGF3 (A), FGF8 (B) and β-catenin (C) RNAs in the presence (yellow) or absence (red) of MOchd, compared with uninjected ichabod embryos (blue) for each. The most common class of embryo partially rescued by β-catenin is dino-like. (D-J) Typical embryos are shown for each indicated condition. |
|
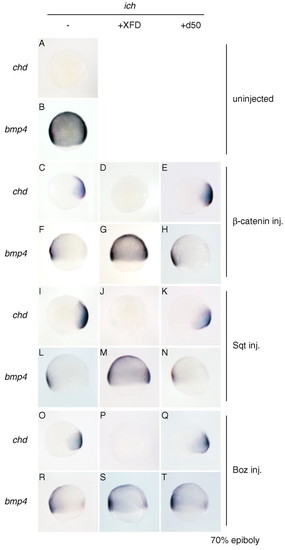
FGF signaling is required for induction of chordin by β-catenin, Squint and Bozozok. chd and bmp4 expression in ichabod embryos A,B) is restored to wild-type pattern by injection of β-catenin RNA (C,F), sqt RNA (I,L), or boz RNA (O,R). Co-injection of XFD RNA inhibits induction of chd by β-catenin (D), Sqt (J) and Boz (P), and dorsal repression of bmp4 by β-catenin (G) and Sqt (M). However, repression of dorsal expression of bmp4 by Boz is observed in XFD RNA-injected embryos (S, compared with R and B). Co-injection with d50 RNA had no effect on β-catenin, Sqt, or Boz functions (E,H,K,N,Q,T). Embryos are at 70% epiboly, shown in lateral view, dorsal to the right. EXPRESSION / LABELING:
|
|
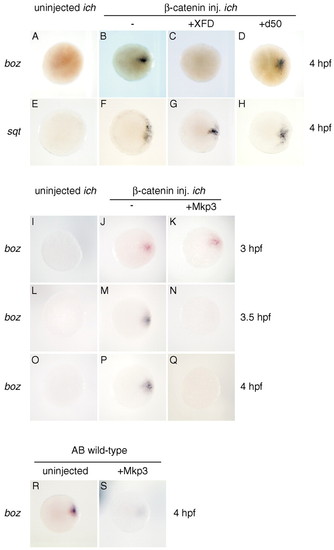
FGF signaling is essential for maintenance but not initial transcription of β-catenin-induced boz RNA. (A-H) FGF signaling is essential for accumulation of β-catenin-dependent boz, but not sqt, expression at 4 hpf. Neither boz nor sqt is expressed in ichabod embryos (A,E), but expression of both genes is restored by injection of β-catenin RNA (B,F). sqt but not boz transcript is expressed in ichabod embryos co-injected with XFD and β-catenin RNAs (C,G), whereas both genes are expressed in embryos co-injected with d50 RNA(D,H). (I-Q) Inhibition of FGF signaling does not affect the initial appearance of boz transcript but affects its maintenance. β-catenin-induced boz transcript can be detected in ichabod embryos at 3, 3.5 and 4.0 hpf (J,M,P), but MKP3 inhibition of FGF signaling results in absence of detectable β-catenin-induced boz RNA at 3.5 and 4.0 hpf (N,Q), although the amount at 3.0 hpf is not reduced (K). (R,S) MKP3 also can inhibit boz RNA accumulation in wild-type embryos. All embryos are shown in animal pole view. |

Unillustrated author statements EXPRESSION / LABELING:
|