- Title
-
nemo-like kinase is an essential co-activator of Wnt signaling during early zebrafish development
- Authors
- Thorpe, C.J., and Moon, R.T.
- Source
- Full text @ Development
|
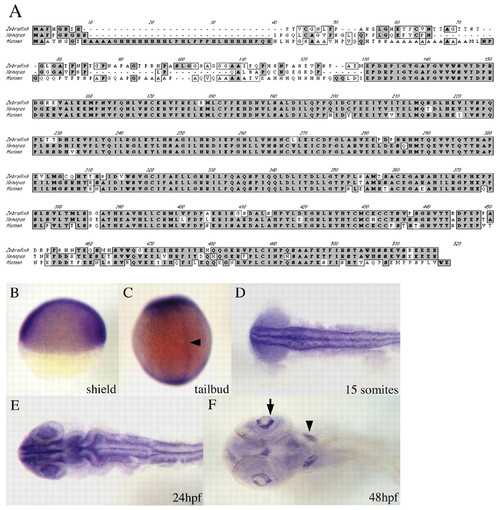
nlk encodes a protein that is highly homologous to other vertebrate Nlks, and is expressed broadly throughout development. (A) CLUSTAL alignment of zebrafish, Xenopus and human Nlk proteins. Identical amino acids are shaded in dark gray; similar residues are in light gray. All three proteins are extremely highly conserved throughout most of the sequence, but are highly divergent near the N terminus. Overall, zebrafish Nlk is most similar to Xenopus Nlk. (B-F) nlk in situ hybridization. (B) Lateral view of a shield stage embryo, dorsal to the right, showing ubiquitous nlk expression. (C) Dorsal view of a tailbud stage embryo. nlk is broadly expressed, but higher levels are seen in the prospective notochord (arrowhead). (D) Dorsal view of a flat-mounted embryo at 15 somites. nlk is highly expressed throughout the CNS, excluding the developing eyes. (E) Dorsal view of a flattened embryo at 24 hours shows intense staining in the eyes, in addition to continued strong staining elsewhere in the CNS. (F) Ventral view of a 48 hpf embryo. nlk transcripts are localized to the otic vesicles (arrowhead), and in the ganglion cell layer of the retina (arrow). |
|
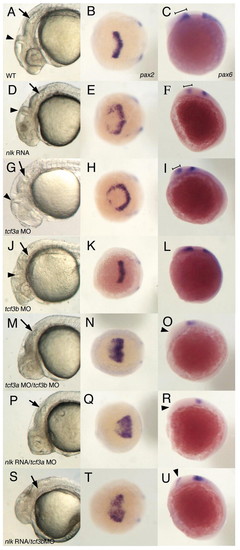
nlk RNA synergizes with tcf3a or tcf3b MOs. Comparison of wild-type (WT; A,B,C), nlk RNA (D,E,F), tcf3a MO (G,H,I), tcf3b MO (J,K,L), tcf3a/tcf3b MOs (M,N,O), nlk RNA/tcf3a MO (P,Q,R) and nlk RNA/tcf3b MO (S,T,U) embryos. nlk RNA (200 pg) was injected in conjunction with 1-2 ng of each of indicated morpholino. Control morpholino (see Materials and methods) was included to balance out the total amount of morpholino injected in each experiment. The first column shows lateral views of the head at 24 hours, with arrows indicating the otic vesicle and arrowheads marking the midbrain/hindbrain boundary (MHB). The middle column shows a dorsal view of embryos fixed at the 2 to 3 somite stage and stained with pax2 probe, whereas the last column shows lateral views of pax6 expression at 3 somites. In nlk RNA (D) and tcf3a MO (G) embryos, the eyes are missing, but embryos still develop a clear MHB, compared with wild type (A). pax2 expression is expanded in both nlk RNA (E) and tcf3a MO (H) embryos. pax6 expression in the diencephalon (marked by brackets in C,F,I) is slightly reduced in nlk RNA (F) and tcf3a MO (I) embryos relative to wild type (C). tcf3b MO embryos show a disorganization of the hindbrain (J), but still make eyes, and have normal pax2 and pax6 expression (K,L). Co-injection of tcf3a and tcf3b MOs results in more severe anterior truncations, with no visible MHB (M), a greatly expanded pax2 expression domain (N), and elimination of diencephalic pax6 (O, arrowhead). Co-injection of nlk RNA with either tcf3a MO (P,Q,R) or tcf3b MO (S,T,U) phenocopies the tcf3a/tcf3b MO phenotype. |
|
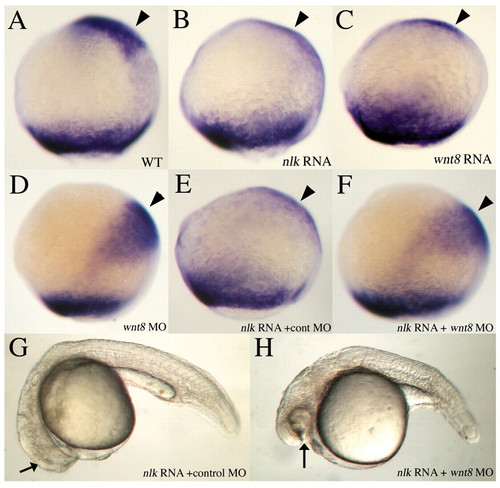
nlk RNA represses anterior neural fate in a wnt8-dependent manner. (A-F) 90-95% epiboly, lateral view, dorsal to the right. Embryos are stained with a mix of opl and tbx6 probes. Injection of 200 pg nlk RNA (B) or 10 pg wnt8 RNA (C) represses expression of opl (arrowheads) in the prospective telencephalon, compared with wild type (A). Injection of 1 ng each of wnt8 ORF1 and ORF2 morpholinos (MOs) causes a posterior shift of opl expression towards the margin (D, arrowhead). Co-injection of a control MO with nlk RNA has no effect on opl (E), whereas injection of wnt8 MOs restores opl expression when co-injected with nlk RNA (F). (G,H) Twenty-four-hour embryos, lateral view, showing rescue of eyes in nlk RNA + wnt8 MOs (G) versus nlk RNA + control MO (H). |
|
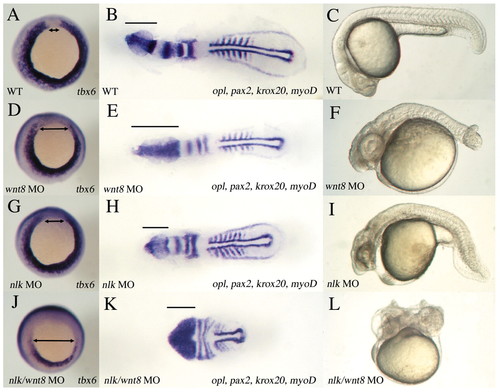
nlk MO enhances the mesoderm defect of wnt8 MOs. (A,B,C) Wild type, (D,E,F) wnt8 MOs, (G,H,I) nlk MO and (J,K,L) nlk MO/wnt8 MOs. A,D,G,J were stained for tbx6 at 90% epiboly (vegetal view; dorsal to the top). B,E,H,K were stained with a mix of opl, pax2, krox20 and myod at 6 to 7 somites (dorsal view; bars indicate the extent of opl expression). C,F,I,L were left to develop to 24 hpf (lateral view). tbx6 expression is reduced in wnt8 (D) and nlk (G) compared with wild type (A), whereas nlk/wnt8 morphants have a dramatic reduction in tbx6 (J; double-headed arrows indicate dorsal region of margin where tbx6 is excluded). wnt8 MO embryos have a greatly expanded opl domain, and a slightly wider notochord (E; notochord domain delineated by longitudinal stripes of adaxial myod expression), reflecting the defects in AP patenting of the neuroectoderm and in DV patterning in the mesoderm, characteristic of wnt8 morphants. nlk morphants (H) are slightly shorter than wild type, but have no significant defects in neuroectoderm or mesoderm patterning. nlk/wnt8 morphants (K) are dramatically shortened, with significantly broadened expression of neural markers, reflecting increased dorsalization of the embryos. Only a few trunk somites are formed, and tail formation does not occur. nlk/wnt8 morphants at 24 hpf (L) are significantly shorter than wild type (C), wnt8 MO (F) or nlk MO (I), and show a dorsal curvature of the tail characteristic of severely dorsalized embryos. |
|
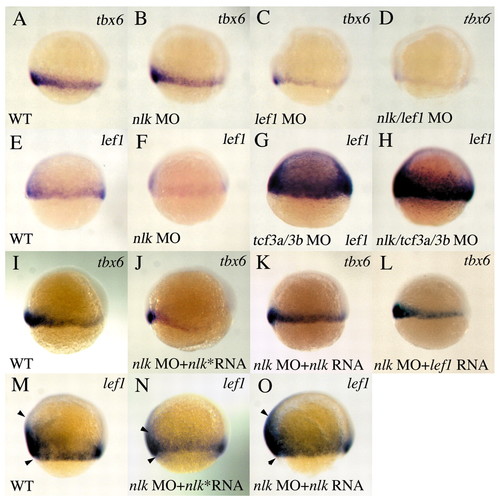
nlk and lef1 cooperatively regulate tbx6, and nlk regulates lef1 expression. (A-D) tbx6 expression at 60% epiboly, lateral view, dorsal to the right. A low dose (0.6 ng) of nlk MO has no effect on tbx6 expression (B, compare with wild type in A). 1 ng lef1 MO reduces tbx6 expression (C), and this effect is enhanced by co-injection of 0.6 ng nlk MO (D). (E-H) Expression of lef1 at shield stage, lateral view, dorsal to the right. lef1 is expressed at the margin in wild type (E), but is reduced in nlk MO (F). lef1 expression is dramatically elevated in tcf3a/tcf3b morphants (G) and in nlk/tcf3a/tcf3b MO embryos (H). (I-L) nlk or lef1 RNA rescues tbx6 expression in nlk morphants. nlk* RNA refers to the C387Y mutant. Co-injection of 200pg nlk RNA (K), but not nlk* RNA (J), restores tbx6 expression to levels similar to wild type (I). 100pg lef1 RNA (L) also rescues nlk morphants. Similarly, nlk RNA rescues normal lef1 expression (O, compare with wild type in M; arrowheads delineate the expression domain), whereas nlk* RNA (N) does not. |
|
nlk MO affects expression of MHB and diencephalon markers. Embryos were injected with nlk or wnt8b MOs. At bud stage, embryos were stained with either a mix of opl, pax2 and tbx6 (A,B,C; dorsal view, anterior to top), or with en2 (D,E,F; dorsal view, anterior to left). At 7 somites, embryos were stained with either en2 (G,H,I; dorsal view, anterior to left) or pax6 (J,K,L; dorsal view, anterior to top). Although expression of opl and pax2 are unaffected in wnt8b morphants (B) compared with wild type (A), nlk morphants have a slight reduction in pax2 expression (C; arrow). en2 expression is reduced in wnt8b MO (E), and is eliminated in nlk MO (F) compared with wild type (D). At 7 somites, en2 is expressed in the MHB and midbrain (G). Expression is substantially reduced in both wnt8b and nlk morphants (H,I; brackets delineate expression domain). pax6 expression is unchanged in wnt8b MO (K), but is reduced slightly in nlk MO (L; brackets indicate AP extent of expression domain). |
|
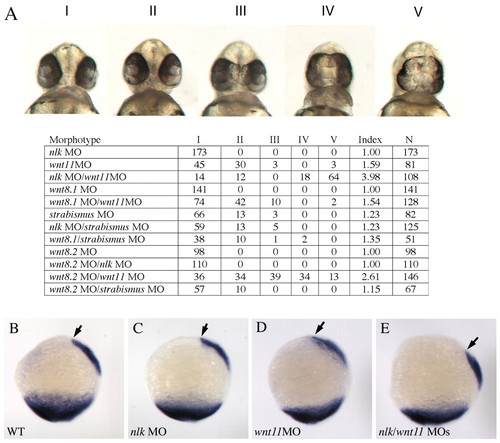
nlk and wnt8 ORF2 MOs enhance the phenotype of wnt11. Morpholinos were injected in the combinations listed in the table. wnt8.1 refers to wnt8 ORF1 and wnt8.2 refers to wnt8 ORF2. Wnt11 and strabismus morpholinos were injected at a concentration of 0.5 ng/nl, whereas nlk, wnt8 ORF1 and wnt8 ORF2 MOs were injected at a concentration of 1 ng/nl. (A) Embryos were scored at 48 hours and placed into five phenotypic classes based on the severity of the cyclopia phenotype (Classes I-V, according to Marlow et al.), and the Cyclopia Index (Marlow et al., 1998) was calculated for each experiment. (B-E) Embryos were injected with nlk, wnt11, or both morpholinos, fixed at 100% epiboly, and stained with probes for tbx6 and gsc. The anterior extent of the gsc domain is marked with an arrowhead. |