- Title
-
Radixin is a constituent of stereocilia in hair cells
- Authors
- Pataky, F., Pironkova, R., and Hudspeth, A.J.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
Demonstration of the specificity of antisera. Each of the 12 illustrations represents an immunoblot incubated with the antiserum indicated below it. The horizontal marker beside each illustration represents 75 kDa. (A) Peptide competition experiments with anti-radixin and anti-ezrin sera on brush border, brain, and cochlear proteins. (Upper) The anti-radixin sera 1041 and 1029 recognize a protein found in the brain and cochlea, but not in brush borders from intestinal epithelial cells. Anti-ezrin serum 1028 reacts strongly with brush-border protein, moderately with brain protein, and weakly with cochlear protein. (Lower) Probing with antisera in the presence of the corresponding peptides used for immunization eliminates immunoreactivity. (B) Immunoprecipitation and blotting of brain and cochlear proteins to demonstrate the specificity of the anti-radixin and anti-ezrin sera. (Upper) Probing the protein immunoprecipitated from the brain by each of the three antisera demonstrates that the anti-radixin and anti-ezrin sera display negligible cross-reactivity. (Lower) The corresponding experiment for proteins immunopreciptated from the cochlea. In each instance, total brain or cochlear protein is loaded in the first lane as a control. |
|
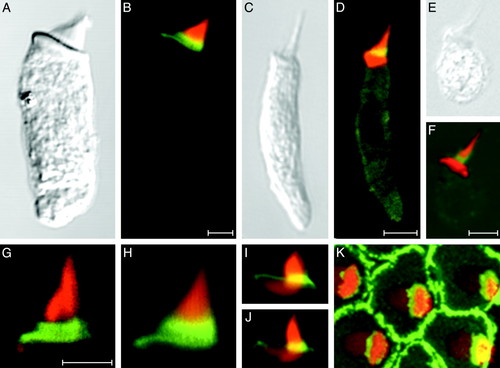
Specific immunocytochemical labeling of radixin in avian hair bundles. (A) A hair cell of intermediate length was isolated from the chicken's cochlea and was observed with differential-interference-contrast optics. (B) Labeling of filamentous actin with phalloidin conjugated to Alexa Fluor 568 (red) produces a strong signal throughout the same hair bundle and the subjacent cuticular plate. The partial overlap of this signal with immunolabeling by anti-radixin (green) results in a yellow signal at the bundle's base. (C and D) Immunolabeling with anti-ezrin produces no signal in a hair bundle labeled with fluorescent phalloidin (red). (E and F) In a negative control experiment, immunolabeling with the anti-radixin antiserum used in B produces a negligible signal in the brush borders of isolated intestinal epithelial cells. (G and H) In a positive control experiment, immunolabeling with the anti-ezrin antiserum used in D robustly labels brush-border microvilli. The cellular nuclei in F and H are weakly stained with TO-PRO-3 iodide (red). (Scale bar in G, 5 μm; applies to all images.) |
|
Anti-radixin immunolabeling of hair bundles from various vertebrate classes. (A and B) A hair cell isolated from the bullfrog's sacculus displays strong anti-radixin labeling (green) at the base of the hair bundle; the yellow signal results from partial overlap of this labeling with that by fluorescent phalloidin (red). (C and D) A hair cell from the lagena of the zebrafish exhibits anti-radixin labeling at the hair bundle's base. (E and F) Anti-radixin immunolabeling occurs at the hair bundle's base in a hair cell isolated from the utriculus of a mouse. (G and H) Higher-magnification views of two hair bundles from the frog's sacculus demonstrate the most intense anti-radixin labeling at the stereociliary bases. (I and J) In higher-magnification views of hair bundles from short hair cells of the chicken's cochlea, anti-radixin labeling is again strongest at the stereociliary bases. The complex shape of the cuticular plate is also apparent. (K) A surface view of a cluster of short hair cells isolated from the chicken's cochlea demonstrates intense anti-radixin labeling of the supporting cells surrounding each hair cell. An additional immunofluorescence signal is evident at the bases of the stereocilia, which are seen in a stack of 73 images acquired at 0.18-μm intervals. All preparations have additionally been labeled with phalloidin conjugated to Alexa Fluor 568 (red). (Scale bars, 5 μm; bar in B applies also to A; bar in D applies also to C, I, J, and K; bar in F applies also to E; bar in G applies also to H.) |
|
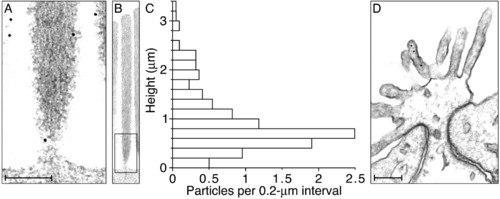
Electron microscopic immunolabeling of anti-radixin activity in the chicken's cochlea. (A) A high-magnification electron micrograph depicts the base of a typical stereocilium bearing two ImmunoGold particles, one on the lower shaft and the other on the stereociliary taper. Additional particles adorn the surfaces of two neighboring stereocilia. (B) A lower-magnification micrograph displays the entire stereocilium whose base is depicted in A; the rectangular box indicates the enlarged region. All immunolabeling in this instance is confined to the lowest 0.6 μm of the stereocilium. (C) Each bin of the histogram depicts the average number of particles per hair bundle encountered in 0.2-μm intervals measured axially along the stereocilia from their basal insertions. A total of 227 gold particles was measured from stereocilia in 22 hair bundles ranging in height from 3.0 to 4.2 μm (3.6 ± 0.3 μm, mean ± SD). All stereocilia were oriented within 15° of the plane of section, so the measurement error owing to obliquity was negligible. Because no attempt was made to compensate for shrinkage during specimen preparation, however, the distance measurements are probably underestimates. (D) An electron micrograph of the apical surface of a supporting cell, which is attached to each of the neighboring hair cells by a junctional complex comprising a tight junction (zonula occludens) and an intermediate junction (zonula adherens). Three ImmunoGold particles occur on one microvillus. (Scale bars in A and D, 0.2 μm; the ordinate of the histogram in C calibrates the aligned micrograph in B.) |