- Title
-
Genetic interactions in zebrafish midline development
- Authors
- Halpern, M.E., Hatta, K., Amacher, S.L., Talbot, W.S., Yan, Y.-L., Thisse, B., Thisse, C., Postlethwait, J.H., and Kimmel, C.B.
- Source
- Full text @ Dev. Biol.
|
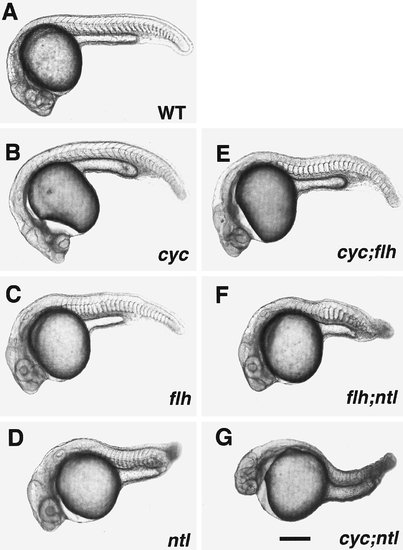
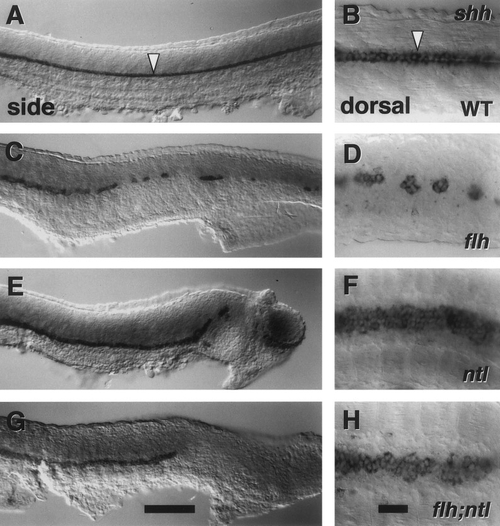
Pharyngula (24 hr) wild-type (WT, A) and mutant embryos (B–G, the gene name abbreviations on this and other figures indicate homozygous mutants for the loci indicated). The head of the cyc;flh double mutant (E) is similar to cyc- (B; see Hatta et al., 1991), whereas the body is similar to flh- (C), with respect to both midline somite fusion and lack of ventral body curvature. The tail of the flh;ntl double mutant (F) is like ntl- (D), and in this example there is a patch of fused somites, as present in flh-, just dorsal to the yolk extension. Somite fusion is variable, occurring in 43% (15/35) of embryos subsequently confirmed to be flh;ntl double mutants by PCR genotyping (see Materials and Methods). The cyc;ntl double mutant (G) combines the cyc- head and ntl- tail phenotypes. Scale bar, 250 um. |
|
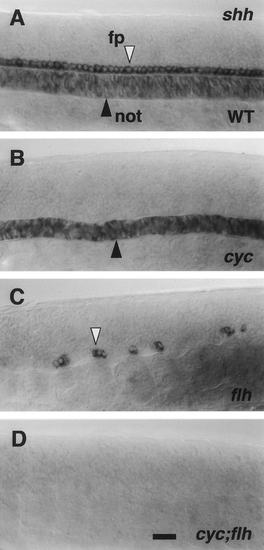
Expression of shh is missing in the trunk midline in cyc;flh double mutants at the late segmentation period. Whole- mounted embryos at 21 hr, left side views, with anterior to the left and dorsal to the top. RNA in situ hybridization. At this stage wild-type embryos (A) the floor plate (fp, white arrowhead) expresses shh strongly, and expression is becoming downregulated in the notochord (not, dark arrowhead), as previously shown by Krauss et al. (1993). The embryos are overstained to reveal weaker levels of notochord shh expression. In cyc mutants (B) only the notochord is labeled (Krauss et al., 1993), in flh mutants (C) only isolated clusters of floor plate cells are labeled (Talbot et al., 1995), and in cyc;flh double mutants (D), there is no labeling at all. Scale bar, 25 um. |
|
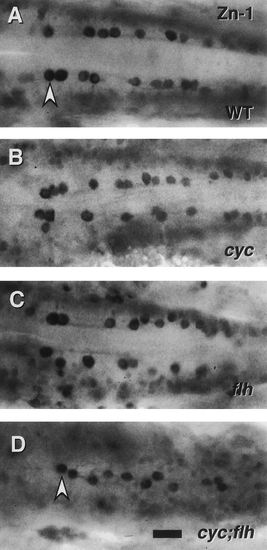
Patterning of ic interneurons is severely disrupted in cyc;flh double mutants. Dorsal views of the caudal hindbrain, with anterior to the left, in whole-mounted embryos labeled with the zn-1 monoclonal antibody at 24 hr. This antibody binds a neuronal antigen that is prominently expressed by ic interneurons, a distinctive class of neurons cells present in the caudal hindbrain that project axons ipsilaterally and caudally, within the medial longitudinal fascicles, to the spinal cord (Mendelson, 1986). In the wild type (A) the ic neurons (arrowhead) line up in bilateral rows adjacent to the medial longitudinal fascicles. The same two cell rows are present (and are closer together) in cyc (B) and flh (C) single mutants. Only a single disorganized row of ic cells is present, just at the midline, in the cyc;flh double mutant (D). Scale bar, 25 um. |
|
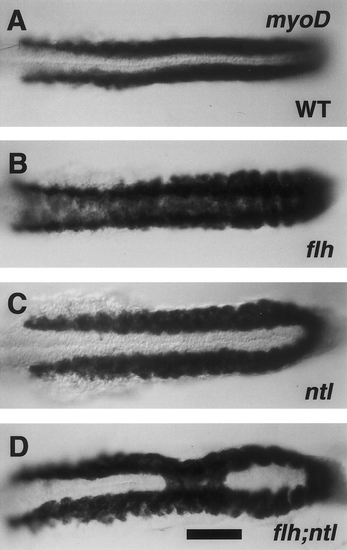
ntl- partially suppresses somitic fusion due to flh-. Dorsal views, with anterior to the left of 19-hr embryos (20-somite stage). RNA in situ hybridization for myoD (Weinberg et al., 1996). In wild-type embryos (A), bilateral stripes of labeling are present in the trunk beside the developing notochord, which is unlabeled at the midline. In flh mutants (B) labeling spreads across the midline, whereas in ntl mutants (C) the stripes are distinctively farther apart than in the wild type (Odenthal et al., 1996). Labeling in the flh;ntl double mutant is generally ntl--like, but a single patch of tissue, involving three adjacent segments, shows the flh--like pattern. Forty-six percent of the double mutants (n = 13) had fusion of myoD expression that included regions of 2 – 5 somites (mean, 3.2 somites; n = 6) at midtrunk levels. The other flh;ntl double mutants were indisinguishable from ntl-. Genotyping of flh alleles was performed on all ntl mutant and flh;ntl mutant embryos by PCR analysis. Scale bar, 100 mm. |
|
ntl- partially suppresses the flh- floor plate defect. Side views, with anterior to the left. The left panels show matched left side views, with dorsal to the top. The right panels show dorsal views at higher magnification. RNA in situ hybridization for shh (Krauss et al., 1993). In wild-type embryos (A, B), a one-cell-wide row of floor plate is labeled along the length of the trunk and tail (arrowheads). The floor plate is disrupted into islands of cells in flh mutants (C, D; 8 of 50 flh mutants had more significant stretches of continuous floor plate labeling than in the example shown). In ntl mutant floor plate is present along most of the length of the trunk, except for the caudalmost region (last 1 – 3 somites, E; we also observed caudal plate to be forked in about half the ntl mutants, and interrupted caudally in 8 of 50). The ntl mutant floor plate is 3 – 4 cells wide (F). Floor plate is present along most of the trunk in flh;ntl double mutants. Compared with ntl mutants, the caudal disruption of the floor plate is more extensive in the flh;ntl double mutant (floor plate is lacking in the caudalmost 4 – 8 somites (G). We also observe local regions along the trunk where the floor plate is disrupted in individual double mutants (not shown). Otherwise, shh labeling in flh;ntl double mutants is generally ntl--like, including substantial floor plate widening (H). We observed the same floor plate phenotypes in these mutants with the marker col2a1 (data not shown). Scale bars, 100 um (left panels), 25 um (right panels). |
|
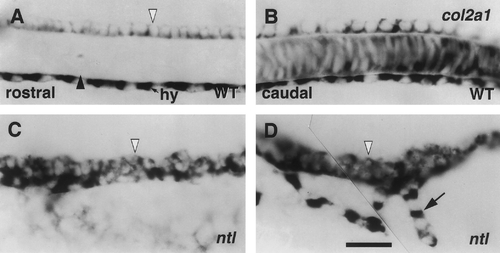
The floor plate is abnormally broad and cell-rich in ntl mutants. Comparisons of expression of col2a1 in wild types (upper panels) and ntl mutants (lower) at rostral (left) and caudal (right) axial levels. Left side views of whole-mounted 24-hr embryos, with anterior to the left and dorsal to the top. RNA in situ hybridization with a col2a1 probe (Yan et al., 1995). Within the spinal cord the wild-type floor plate is a 1-cell-wide row (A, B, white arrowhead). Notochord (dark arrowhead) and hypochord (hy) also express the marker. Often the floor plate in the ntl mutant trunk appears irregular (C, D), in a row several cells wide. In addition, ventral projections of cells with the col2a1 expression characteristic of hypochord (arrow) are often seen in the ntl mutant caudal trunk (D). However, expression of radar, a marker characteristic of the hypochord in wild-type embryos, was not detected in ntl mutants by Rissi et al. (1995). Scale bar, 25 um. |
|
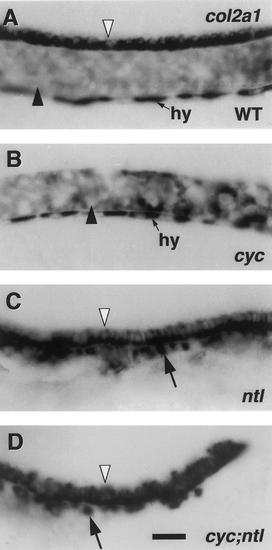
Floor plate but not notochord is present in cyc;ntl double mutants. Left side views, with anterior to the left and dorsal to the top of whole-mounted 24-hr embryos. RNA in situ hybridization with a col2a1 probe (Yan et al., 1995). In the wild-type (A) expression is in the notochord (dark arrowhead), in the single row of cells that comprise the floor plate (white arrowhead), and in the hypochord (hy). The floor plate is specifically missing in cyc- (B). The notochord and possibly hypochord (Rissi et al., 1995) are missing in ntl- (C), whereas the floor plate is present (arrowhead), as are col2a1-expressing cells just ventral to the spinal cord and floorplate (arrow). Floor plate is also present in the cyc;ntl double mutant hindbrain and trunk spinal cord (D) but labeling does not extend as far caudal as in ntl mutants. Cells beneath the floor plate also label in double mutants but these are fewer in number than in ntl single mutants (arrow, compare with C). Scale bar, 25 um. |
|
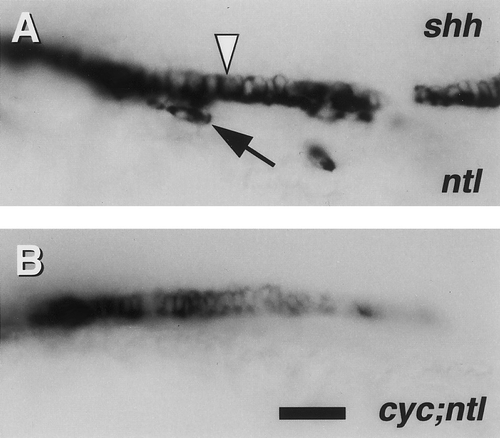
Midline expression of shh in ntl mutants (A) and cyc;ntl double mutants (B) detected by RNA in situ hybridization. Left side views of the trunk of whole-mounted 24-hr embryos, with anterior to the left and dorsal to the top. In ntl mutants, shh transcripts are present in floor plate (white arrowhead) and in a few cells beneath the spinal cord (arrow). cyc;ntl double mutants only show floor plate labeling, present in approximately a 1-cell-wide row. Scale bar, 25 um. |
|
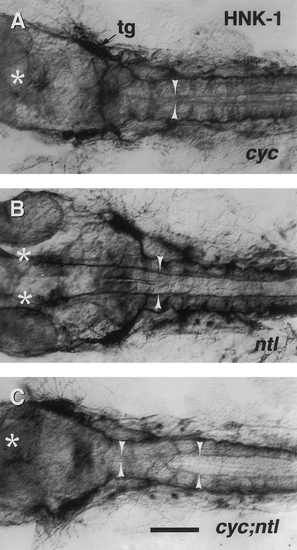
Floor plate-dependent axonal pathfinding is intact in the caudal hindbrain of cyc;ntl double mutants. Dorsal views, with anterior to the left, of the caudal head region of 30-hr embryos, labeled with the zn-12 monoclonal antibody (Trevarrow et al., 1990). This antibody binds the HNK-1 epitope broadly expressed on developing neurons (Metcalfe et al., 1990), including the medial longitudinal fascicles (MLF, arrowheads) which emanate from bilateral neuronal clusters in the midbrain, the nuclei of the MLF (nMLF, asterisks). The MLFs collect additional axons from hindbrain neurons (Metcalfe et al., 1986; Hatta, 1992). The trigeminal ganglion (tg) is approximately at the level of the midbrain – hindbrain boundary. In cyc mutants (A) the normally paired nMLF clusters form a single unpaired midline cluster and the MLF axons are poorly defasciculated, present in or near the midline, and often cross the midline (Hatta, 1992). In contrast, in ntl mutants (B) the nMLF positions and the MLF axonal trajectories are as in wild-type embryos described previously (Hatta, 1992), and the MLF axons are likewise well-fasciculated. In the midbrain and rostral hindbrain, cyc;ntl double mutants (C) exhibit defects similar to cyc mutants. Correct axonal pathfinding and axonal fasiculation, however, recovers in the hindbrain, the level approximately corresponding to the most rostral region where floor plate is present in these mutants. Scale bar, 100 um. |
|
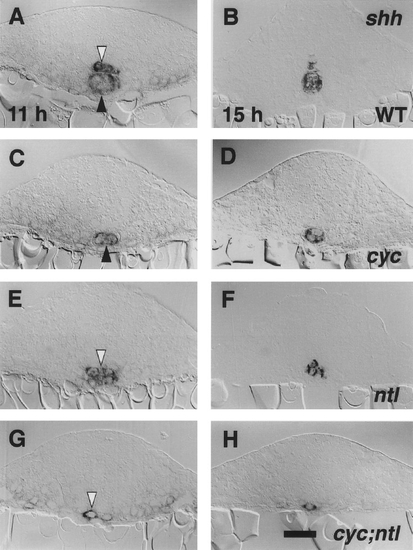
Cells expressing shh are present in a 1-cell-wide row in the trunk midline of cyc;ntl double mutants. Transverse sections with dorsal to the top, through 11-hr embryos (4- to 6-somite stage, right panels) and 15-hr embryos (11- to 12-somite stage, left panels). Whole- mount RNA in situ hybridization (Krauss et al., 1993) and epon sections of 7 um were prepared as described under Materials and Methods. shh is expressed the floor plate region (white arrowheads) and in the notochord primordium (dark arrowheads) at these stages in wild- type embryos (A, B). Only the notochord is labeled in cyc mutants (C, D). In ntl mutants (E, F), it was often difficult to assign the position of labeled cells as being within or just beneath the developing spinal cord. Generally only a single labeled cell is observed at the same location in cyc;ntl double mutants (G, H). We quantified the difference between ntl and cyc;ntl mutants by counting shh-expressing cells in the midline of sections through the trunk at 11 hr. The number of shh-expressing cells per section was determined to be 3.6 ± 0.4 (mean ± SEM) for ntl mutants (n = 3 embryos, 86 sections total) and 1.2 ± 0.1 for cyc;ntl mutants (n = 3 embryos, 65 sections). The difference is significant (P < 0.05; from a t test assuming unequal variances). Scale bar, 25 um. |
Reprinted from Developmental Biology, 187(2), Halpern, M.E., Hatta, K., Amacher, S.L., Talbot, W.S., Yan, Y.-L., Thisse, B., Thisse, C., Postlethwait, J.H., and Kimmel, C.B., Genetic interactions in zebrafish midline development, 154-170, Copyright (1997) with permission from Elsevier. Full text @ Dev. Biol.