- Title
-
Ultraviolet irradiation impairs epiboly in zebrafish embryos: evidence for a microtubule-dependent mechanism of epiboly
- Authors
- Strähle, U., and Jesuthasan, S.
- Source
- Full text @ Development
|
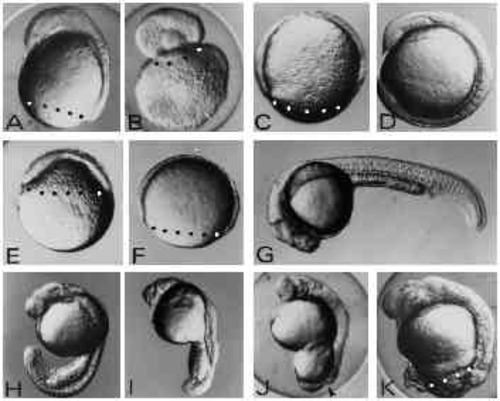
Effects of UV exposure on zebrafish embryos. (A-D) The three classes of embryos generated by UV exposure of zygotes (A-C) and an untreated control embryo (D) at 12 hours of development. In the type-A embryo (A) the blastoderm has covered the yolk cell up to 80% and shows a well-formed anterior axis rudiment on the dorsal side. In type-B embryos (B) the blastoderm rounded up forming a vesicle on top of the yolk mass. Type-C embryos (C) show retardation of epiboly but do not form an axis rudiment. (E,F) UV-treated (E) and control embryo (F) at 8 hours of development. UV treatment results in strong retardation of epiboly. Control embryos (F) are at the 80% epiboly stage, whereas UV-exposed embryos (E) have just covered the yolk sphere by 40% at 8 hours after fertilisation. (G-K) UV-irradiated embryos (H-K) and a control embryo (G) at 24 hours of development. UV-treated embryos at this stage show various degrees of posterior defects ranging from subtle tail defects (H,I) to complete failure to finish epiboly (J,K) leaving the yolk cell uncovered by the blastoderm at the vegetal pole. The arrowhead in J indicates the open blastopore. Embryos were exposed to 1.8 mJ/cm2 at the late zygote stage. Blastoderm margins are indicated by black or white dots. Embryos are oriented anterior up and dorsal to the right with the exception of panel G where anterior is to the left and dorsal up. |
|
Retardation of epiboly by UV exposure is stageindependent. Embryos at 16-cell (A) or 50% epiboly stage (B) were exposed to UV light (2.7 mJ/cm2) and cultivated until untreated control embryos (C) had reached the 4-somite stage. Blastoderm margin is indicated by dots. The embryo in B has just closed the blastopore (pointed out by an arrowhead). Embryos are oriented anterior up and dorsal to the right. |
|
Initiation of gastrulation is independent of the degree of epiboly. In this UV-treated embryo at the 30 % epiboly stage, a labeled involuted cell (arrowhead) is seen migrating upwards towards the animal pole. Untreated controls (not shown) were at the 50% epiboly at this time and had also begun involution. The frames shown were taken at 10 minute intervals. The embryos were irradiated at early cleavage stages and then labeled by injecting a single blastomere at the 32-cell stage with TRITC-dextran. Animal poles are up, the dorsal side could not be identified at these early involution stages. |
|
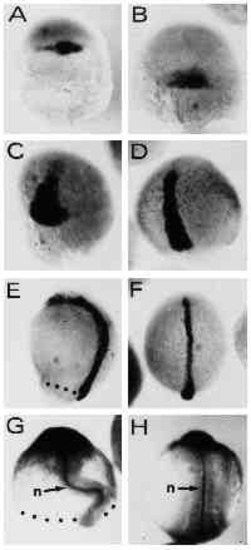
Expression of dorsal gene markers is unaffected by UV treatment in type-A embryos. Embryos were hybridized with a digoxigenin-labeled antisense RNA probe complementary to the Axial gene RNA (A-F) or stained with the anti-ntl (zebrafish Brachyury, ZF-T) antibody (G,H). (A,B) UV-retarded embryo at 30% epiboly and control embryo at 50% epiboly stage, respectively. Axial expression marks the region of the fish organizer. (C,D) UV embryo at 50 % epiboly and untreated control at 80% epiboly, respectively. Axial -postive cells have involuted and spread towards the animal pole along the dorsal midline of the embryos. (E,F) UV-retarded and control embryo, respectively. Control embryo is at the 4-somite stage. (G,H) 14- hour embryos stained with the anti-ntl antibody. Embryo in G was UV treated. (H) An untreated control embryo. The notochord is indicated by n. Embryos were UV treated at early cleavage stages (3.6 mJ/cm2). Blastoderm margins are indicated by dots. Orientation of embryos is anterior up. |
|
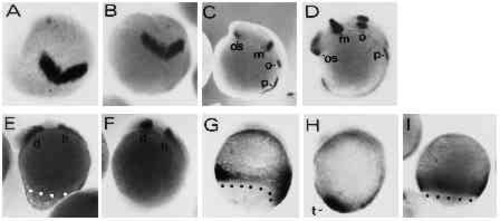
Regional specification of the body axis in type-A embryos appears to be unaffected by the UV treatment. UV retarded type-A and control embryos were stained with various digoxigenin-labeled RNA probes to assess the regional specification in the truncated axes. (A,B) UV-treated and control embryos at the 2-somite stage stained with an antisense RNA probe directed against the Zf[pax-b] gene. Expression of Zf[pax-b] is confined to the anlage of the mesencephalon in the neural plate at the 2-somite stage. View onto animal pole is shown, dorsal is at the bottom. (C,D) UV-treated and control embryo at the 8-somite stage labelled with the Zf[pax-b] antisense probe. os, optic stalk; m: mesencephalon; o: otic vesicle; p: pronephros. (E,F) UV-treated and untreated control embryo stained with the ZF[pax-A]. gene. Staining in the diencephalon and the hindbrain is marked by d and h, respectively. (G,H) UV-treated and control embryo at the 4- somite stage. Embryos were stained with the ZFcad1 probe. t points out the tail bud in control embryo. (I) An 80% epiboly control embryo stained with ZFcad1 for comparison with staining in G. Embryos, with exception of A and B, are oriented anterior up and dorsal to the right. Blastoderm margins are highlighted by black or white dots. UV embryos were exposed to 3.6 mJ/cm2 UV. |
|
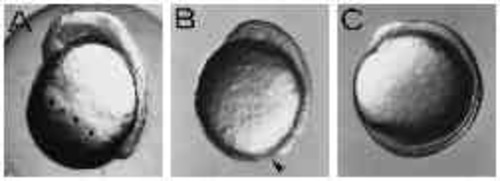
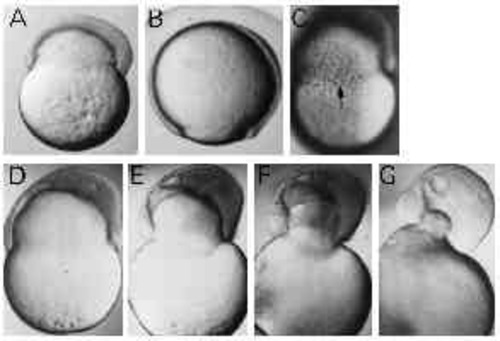
Type-B embryos constrict off the yolk. (A,B) UV-treated and control embryo of the same age, respectively. UV-treated embryo shows constriction of the yolk cell. Dorsal to the right, anterior up. (C) Circumferentially elongated cells at the margin of the blastoderm in a Type-B embryo (arrow). (D-G) The detachment of the blastoderm from the yolk mass. The frames were taken within 30 minutes. |
|
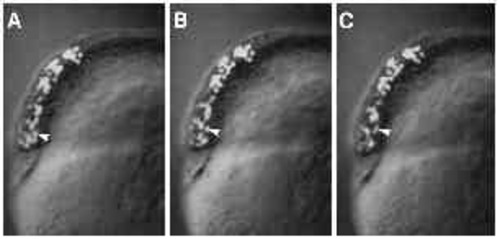
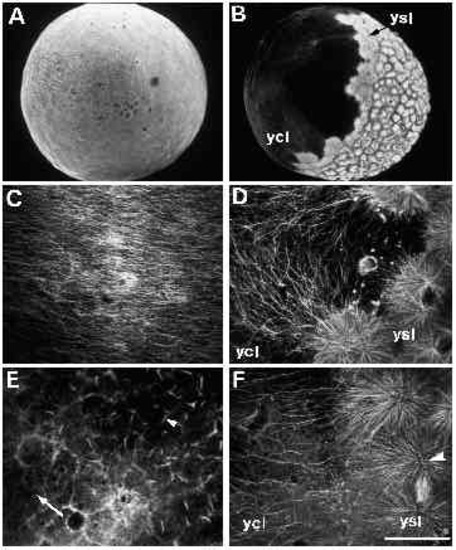
Immunofluorescent staining of microtubules in untreated (panels A-D) and UVtreated embryos (panels E-F). (A) An untreated embryo, approximately 30 minutes after fertilization. When examined at high magnification (C), a parallel array of microtubules can be seen in the yolk cytoplasmic layer. (B) An untreated embryo at approximately 3.5 hours after fertilization, with a spreading yolk syncytial layer (ysl). Numerous microtubules radiate from the yolk syncytial layer (ysl) into the yolk cytoplasmic layer (ycl), as shown at high m a g n i fication in D. Microtubules are disrupted in UV-treated embryos (E,F). In embryos fix e d 10 minutes after irradiation, microtubules throughout the embryo appear abnormal. (E) One such embryo, where microtubules in the yolk cytoplasmic layer are either very sparse (arrow) or present in the aberrant form of ‘comet-tails’ (arrowhead). In embryos fixed 2 hours after irradiation, an example of which is shown in F, some microtubules appear normal, as demonstrated by the mitotic aster in the yolk syncytial layer (arrowhead). In the yolk cytoplasmic layer, however, microtubules are still disrupted: there are far fewer microtubules in the region bordering the yolk syncytial layer (compare with similar region in panel D). Bar, 250 7mu;m (for A,B); 50 μm (for C-F). |
|
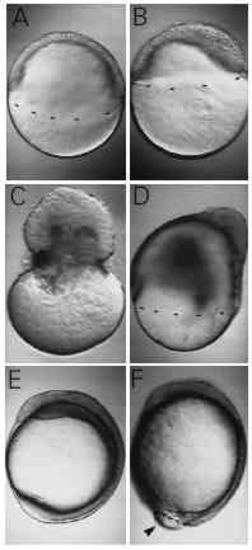
Nocodazole, a microtubule-depolymerizing agent, mimics the effect of UV. (A) An untreated embryo at 50% epiboly. A sibling, incubated in 1 μg/ml nocodazole, has reached only 30% epiboly (B). (C,D) Observations made at a later stage, when controls have just completed epiboly. 2 μg/ml of nocodazole caused the blastoderm to pinch off the yolk cell (C), while 1 μg/ml caused severe retardation of epiboly (D). At 12 hours after fertilization, when untreated embryos have long finished epiboly (E), an embryo treated with 0.6 μg/ml has still not closed the blastopore (F, arrowhead). Although epiboly is retarded, a distinct axis has formed, with somitogenesis well underway. |