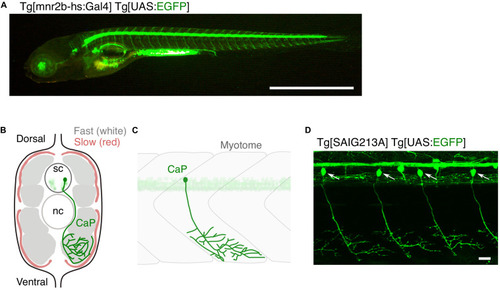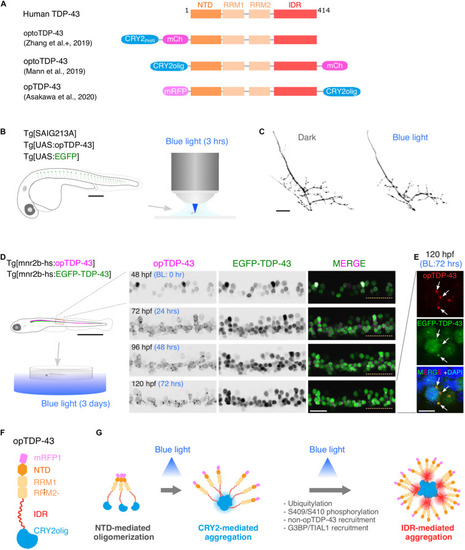
Optogenetic induction of TDP-43 aggregates in in vivo spinal motor neurons. (A) Structures of optogenetic TDP-43. The human TDP-43 (top) consists of 414 amino acid residues subdivided into the N-terminal domain (NTD), 2 RNA recognition motifs (RRM1 and RRM2), and a C-terminus intrinsically disordered region (IDR). The optoTDP-43 (Mann et al., 2019; Zhang et al., 2019) and opTDP-43 (Asakawa et al., 2020) constructs carry the CRY2-modules at their N- and C-terminus, respectively. mCherry (237 aa), mRFP1 (225 aa), CRY2PHR (498 aa), and CRY2olig (498 aa) not to scale with TDP-43. (B) An agarose-embedded zebrafish embryo expressing both EGFP and opTDP-43 in CaPs (Tg[SAIG213A] Tg[UAS:opTDP-43] Tg[UAS:EGFP] triple transgenic) is illuminated with a confocal blue laser light for 3 h, 28–31 h post-fertilization (hpf). (C) The total axonal length at 48 hpf was reduced in the CaP irradiated with the blue light (Blue light) compared to the CaP grown in the dark (Dark). The figure panels are adapted from Asakawa et al. (2020). (D) An unrestrained zebrafish larva expressing both opTDP-43 and non-optogenetic EGFP-TDP-43 was irradiated with blue LED light. The spinal motor column was scanned every 24 h for 3 days (from 48 to 120 hpf). The duration of the blue light illumination is indicated in blue letters. Horizontal dashed line demarcates the ventral limit of the spinal cord. The figure panels are adapted from the study by Asakawa et al. (2020). (E) Cytoplasmic opTDP-43 foci colocalize with non-optogenetic EGFP-TDP-43 (arrows) in the spinal motor neurons at 120 hpf in (D). BL, Blue light. (F) Schematic drawing of opTDP-43 protein. (G) Blue light illumination drives CRY2olig-dependent opTDP-43 oligomerization and aggregation. A short-term illumination induces the oligomerization of opTDP-43, whereas a long-term illumination causes cytoplasmic aggregation of opTDP-43. Non-optogenetic TDP-43 is incorporated into the opTDP-43 aggregates [e.g., EGFP-TDP-43 in (E)] possibly through IDR-mediated intermolecular interactions. Cytoplasmic opTDP-43 aggregates are partially positive for immunoreactivities against ubiquitin, phosphorylation at S409/S410, and classical stress granule components (G3BP and TIAL1) (Asakawa et al., 2020). The bars indicate 250 μm (B), 20 μm (C), 1 mm (D, left), 20 μm (D, panels), and 5 μm (E).
|