- Title
-
Extracellular Pgk1 enhances neurite outgrowth of motoneurons through Nogo66/NgR-independent targeting of NogoA
- Authors
- Lin, C.Y., Wu, C.L., Lee, K.Z., Chen, Y.J., Zhang, P.H., Chang, C.Y., Han, H.J., Lin, S.Z., Tsai, H.J.
- Source
- Full text @ Elife
|
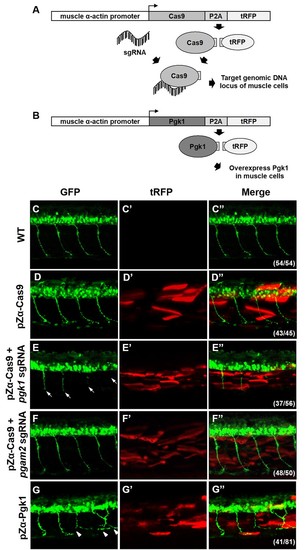
Muscle-specific overexpression of Pgk1 enhances NOM in zebrafish embryos.(A) Diagram of plasmid pZα-Cas9. The Cas9-P2A-tRFP cassette is driven by zebrafish muscle-specific α-actin promoter. The fusion protein Cas9-P2A-tRFP expressed in muscle cells is digested into Cas9, which is bound by transferred sgRNA, resulting in silencing the target gene in muscle cells. (B) Diagram of plasmid pZα-Pgk1. Pgk1 is specifically overexpressed in muscle cells. (C–G) Injection of different materials, as indicated, into embryos from transgenic line Tg(mnx:GFP) and observation of fluorescent signals expressed in embryos at 30-hpf. GFP-labeled motor neurons observed under confocal microscopy. (C’–G’) Location of RFP-labeled muscle cells in which Cas9 and/or Pgk1 is overexpressed. (C’’–G’’) Two fluorescent signals were merged. Numbers shown in the lower right corner were the number of phenotypes out of total examined embryos. (C–C”) Untreated embryos served as the control group. (D–D”) Injection of pZα-Cas9. NOM was not affected. (E–E”) Injection pZα-Cas9 combined with pgk1 sgRNA. The length of NOM became shorter (white arrows). (F–F”) Injection of pZα-Cas9 combined with pgam2 sgRNA (served as negative control). The NOM was not affected. (G–G”) Injection of pZα-Pgk1. The NOM became increasingly ectopic toward the muscle cells in which Pgk1 was overexpressed (white arrowheads). PHENOTYPE:
|
|
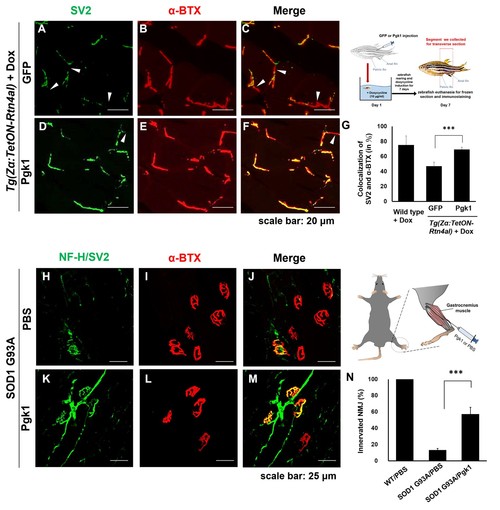
Pgk1 rescues denervation caused by NogoA-overexpression in the muscle cells of zebrafish, as well as ALS mouse model. NMJ phenotype of transgenic zebrafish Tg(Zα:TetON-Rtn4al) harboring Rtn4al (NogoA homolog) cDNA driven by a Dox-inducible muscle-specific α-actin promoter after intramuscular injection of (A–C) GFP protein control and (D–F) Pgk1. Axons were labeled by synaptic vesicle glycoprotein 2A (SV2) in green, while postsynaptic receptors were labeled by α-Bungarotoxin (α-BTX) in red. (G) Statistical analysis of the number of colocalized axons and postsynaptic receptors in muscle of NogoA-overexpression zebrafish ALS-like model using Student’s t-test (***, p<0.001). NMJ phenotype of ALS mouse model harboring human SOD1 G93A after intramuscular injection of (H–J) PBS and (K–M) Pgk1 in the gastrocnemius muscle of the right hind leg. Neurofilament-H (NF-H) and SV2 labeled by green fluorescent signal were used to detect the axon terminal of motoneurons, while α-BTX labeled by red fluorescence signal was used to detect the acetylcholine receptor on motor endplates. (N) Statistical analysis of the number of innervated NMJ among PBS-injected WT mouse, PBS-injected SOD1 G93A mouse and Pgk1-injected SOD1 G93A mouse. Statistical analysis used Student’s t-test (***, significant difference at p<0.001). |
|
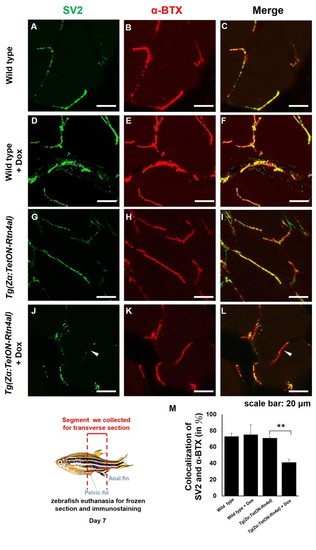
Overexpression of Rtn4al in adult zebrafish muscle causes NMJ denervation.(A–C) Water-immersed WT, (D–F) Dox-immersed WT, (G–I) water-immersed Tg(Zα:TetON-Rtn4al), and (J–L) Dox-immersed Tg(Zα:TetON-Rtn4al). Adult fish of all groups were treated for one week before histopathological examination. (A–L) Transverse section. (A, D, G, J) Green fluorescent signal detection of synaptic vesicle glycoprotein 2A (SV2) labeled in the peripheral motor neuron. (B, E, H, K) Red fluorescence signal using α-Bungarotoxin (α-BTX)-labeled acetylcholine receptor on motor endplate. (C, F, I, L) comprised the merge picture. Arrowhead indicates the location of NMJ denervation. Scale bar, 20 μm. (M) Four experimental groups of SV2 and α-BTX signal colocalization percentage quantitative data. Data from three adult fish from each experimental group; three images each magnified at 600X, using Metamorph software to quantify SV2 and α-BTX signal colocalization area (400 × 400 pixels), followed by conversion to percentage after Student’s t-test and used for statistical analysis (**, significant difference at p<0.01). PHENOTYPE:
|
|
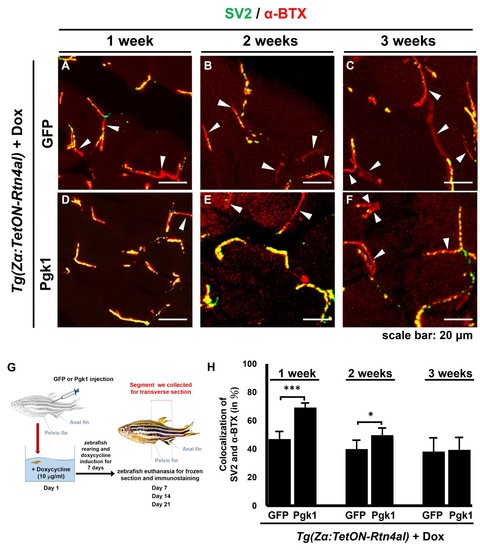
Administration of Pgk1 could delay NMJ denervation caused by overexpression of Rtn4al in adult zebrafish muscle.NMJ phenotype of transgenic zebrafish Tg(Zα:TetON-Rtn4al) harboring Rtn4al/NogoA cDNA driven by a Dox-inducible muscle-specific alpha-actin promoter was observed after intramuscular injection of (A–C) GFP protein (served as a control) and (D–F) Pgk1 protein. Histopathological examination of the muscle tissue around the injection site of adult fish was performed after fish were treated for one, two and three weeks as indicated. (A–F) Transverse section. Green fluorescent signal was used to detect synaptic vesicle glycoprotein 2A (SV2)-labeled peripheral motor neuron, while red fluorescence signal was used to detect α-Bungarotoxin (α-BTX)-labeled acetylcholine receptor on motor endplate. Arrowheads indicate the location of NMJ denervation. Scale bar, 20 μm. (G) Diagram depicts places where GFP and Pgk1 were injected and where the muscle samples were taken after soaking with Dox in adult transgenic zebrafish Tg (Zα:TetON-Rtn4al). (H) Quantitative analysis of the colocalization of SV2 and α-BTX signals. Data were averaged from three adult fish. Each fish datum was averaged from three images, whereas each image was counted by using Metamorph software to quantify SV2 and α-BTX signal colocalization area (400 × 400 pixels) from 600X magnification, followed by conversion to percentage. Student’s t-test was used for statistical analysis (***, significant difference at p<0.001; *, p<0.05). |