- Title
-
A novel technique based on in vitro oocyte injection to improve CRISPR/Cas9 gene editing in zebrafish
- Authors
- Xie, S.L., Bian, W.P., Wang, C., Junaid, M., Zou, J.X., Pei, D.S.
- Source
- Full text @ Sci. Rep.
|
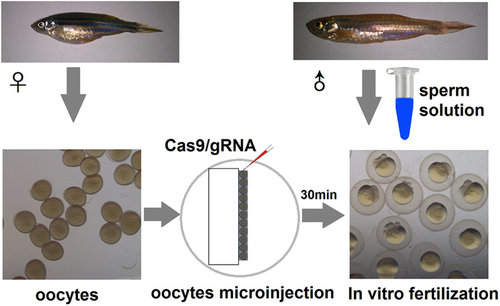
The schematic diagram of microinjection and oocyte storage. |
|
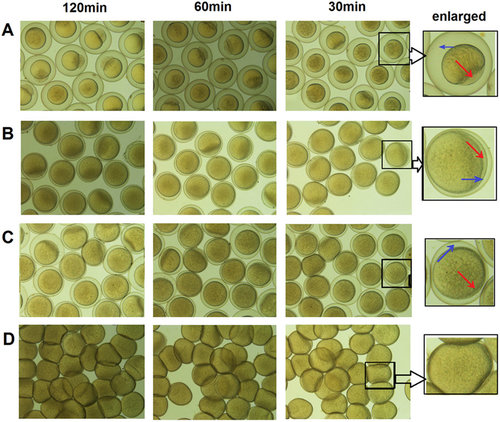
The preservation effects of three different oocyte storage media. (A) Fresh water, (B) Hepes-cortland medium, (C) 90% L-15 medium without BSA at pH 9.0, (D) 90% L-15 medium with BSA at pH 9.0. The storage time in three oocyte storage media was 30 min, 60 min and 120 min, respectively. The cavities between the shell and vitelline membrane and the animal pole were indicated with red and blue arrows. |
|
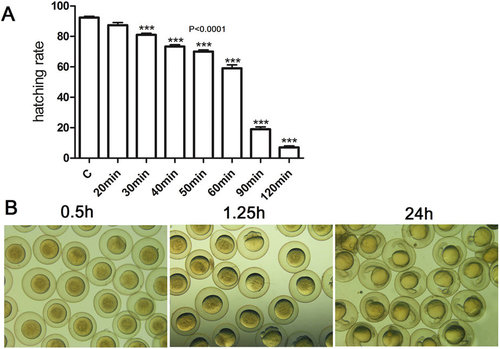
Effects of storage time on hatching rates and embryonic development in 90% L-15 medium with BSA at pH 9.0. (A)The relationship between the hatching rate and the storage time in vitro. The oocytes were stored in oocyte storage medium for different time and then fertilized in vitro. The hatching rates were recorded after 72 h. (B) The embryos developed normally when oocytes were stored for 30 min in vitro. The oocytes were stored in oocyte storage medium for 30 min and then fertilized in vitro. The embryonic development were observed from 0.5 h to 24 h. |
|
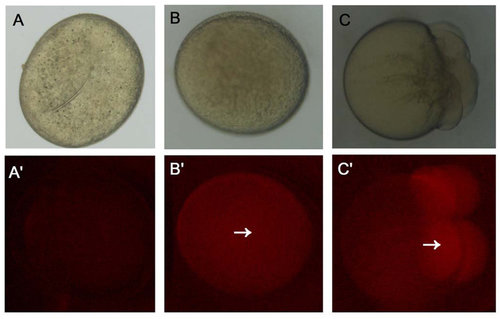
The translational capacity of mature oocytes. (A) Oocytes were stored for 2 h in vitro without injecting mCherry capped RNAs. (B) Oocytes were stored for 2 h in vitro and micro-injected with mCherry capped RNAs. (C) Fertilized eggs at 1 cell stage were micro-injected with mCherry capped RNAs after 2 h. The red fluorescence was indicated with white arrows. |