- Title
-
CSF-contacting neurons regulate locomotion by relaying mechanical stimuli to spinal circuits
- Authors
- Böhm, U. L., Prendergast, A., Djenoune, L., Nunes Figueiredo, S., Gomez, J., Stokes, C., Kaiser, S., Suster, M., Kawakami, K., Charpentier, M., Concordet, J. P., Rio, J. P., Del Bene, F., Wyart, C.
- Source
- Full text @ Nat. Commun.
|
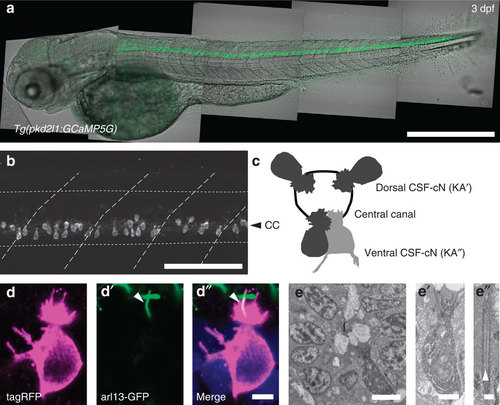
In the spinal cord, pkd2l1+CSF-cNs project an apical extension consisting of one kinocilium and a brush of microvilli into the central canal. (a) Transmitted and fluorescent image showing that the pkd2l1 promoter in Tg(pkd2l1:GCaMP5G) transgenic larva drives GCaMP5G expression in CSF-cNs along the entire spinal cord. Scale bar: 500 µm. (b) A close up from a lateral view in the same transgenic animal shows the morphology of CSF-cNs as elongated cells with an apical extension reaching the central canal. Scale bar: 100 µm. (c) Schematic depicting dorsal and ventral CSF-cN location around the central canal. (d) Confocal microscopy of 50 µm sections of the 4 dpf triple transgenic larva Tg(pkd2l1:gal4, UAS:tagRFP-CAAX;cmcl2:GFP, βact:Arl13-GFP) shows that CSF-cNs project one Arl13-GFP+ cilium (arrowhead) and multiple microvilli into the CSF. Scale bar: 3 µm. (e) Transmission electron microscopy in Tg(pkd2l1:Gal4) larvae injected with UAS:APEX2-tagRFP shows a single ventral CSF-cN reaching the central canal (close up in (e′), scale bar: 2µm) and exhibiting a motile 9+2 cilium (arrowhead, (e′′), scale bar: 250 nm). EXPRESSION / LABELING:
|
|
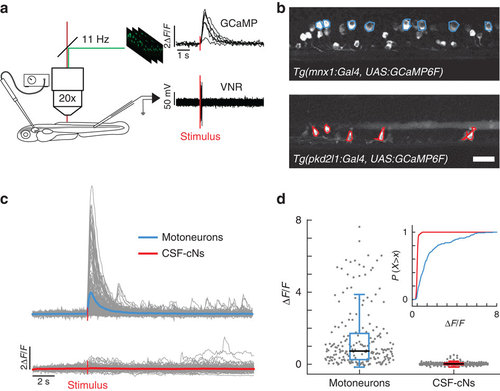
CSF-cNs are minimally activated during fictive escapes when no muscle contraction occurs. (a) Schematic view of the experimental setup combining 2-photon laser scanning microscope for calcium imaging and electrophysiological recordings of the ventral nerve root. 10 ms-long water jets delivered to the otic vesicle triggered fictive escapes in paralyzed larvae. (b) Lateral view showing expression of GCaMP6f in MNs in the double transgenic larva Tg(mnx1:Gal4, UAS:GCaMP6f;cryaa:mCherry) and in CSF-cNs in Tg(pkd2l1:gal4, UAS:GCaMP6f;cryaa:mCherry). ROIs indicate cells included in the analysis. Only the dorsalmost MNs were analyzed in the mnx1 line. Scale bar: 20 µm. (c) Typical calcium transients recorded in MNs (blue) and in CSF-cNs (red) during fictive escapes; ‘stimulus’ indicates when the water jet was triggered, average response in coloured lines. (d) Quantification of calcium transient amplitude in MNs and CSF-cNs (each data point represents one recording from one cell; plots use median as the measure of central tendency; inset is the cumulative histogram of calcium responses). Responses in both populations are greater than baseline (204 MNs from 6 larvae: mean ΔF/F=1.2, P<1.0 × 10-8; 192 CSF-cNs from 7 larvae: mean ΔF/F=0.042, P=3.78 × 10-8), but CSF-cNs exhibit significantly less activity than MNs (P=0.014). |
|
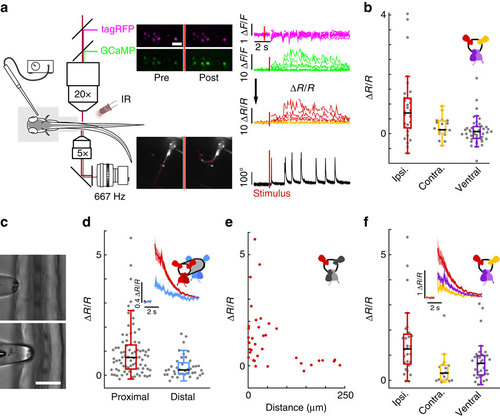
CSF-cNs respond to active muscle contraction as well as to passive mechanical bending of the spinal cord. (a) Schematic describing 2-photon imaging experiments used to record simultaneously from CSF-cNs expressing tagRFP (magenta) and GCaMP5 (green) in head-embedded Tg(pkd2l1:GCaMP5, pkd2l1:tagRFP) larvae. Infrared illumination combined with high-speed video recording shows unidirectional tail deflections induced by a water jet to the otic vesicle. Sample traces for ΔF/F of tagRFP and GCaMP shown with tail deflection during escape (note: vertical scale is 10 times larger for GCaMP compared to tagRFP signals). Subtracting the tagRFP signal from the GCaMP signal removed motion artifacts; breaks in the trace arise from frames when cells escaped from the focal plane. Scale bar: 10 µm. (b) Quantification of calcium transient amplitude in response to muscle contraction (n=11 larvae) in dorsal CSF-cNs either ipsilateral (red, 31 cells) or contralateral (yellow, 19 cells) or ventral (purple, 44 cells). Only dorsal ipsilateral cells exhibited responses greater than baseline (P=9.51 × 10-4) and all other cell types responded significantly less than dorsal ipsilateral cells (dorsal contralateral: P=2.43 × 10-3, ventral: P=4.85 × 10-5). (c) Passive mechanical stimulation of CSF-cNs in paralyzed larvae (n=5) was implemented with mechanical pressure exerted by pushing a glass probe laterally against the fish tail. Scale bar: 50 µm. (d) Response of proximal (<100 µm) and distal (>100 µm) CSF-cNs. Inset: average calcium response of proximal (red) versus distal (blue) CSF-cNs. (e) Response of dorsal ipsilateral (red) CSF-cNs as function of distance from the probe. (f) Response of dorsal ispsilateral (red, 28 cells), dorsal contralateral (yellow, 16 cells) and ventral (purple, 36 cells) CSF-cNs relative to the location of mechanical stimulation. Inset: Average calcium response of dorsal ispsilateral versus dorsal contralateral and ventral CSF-cNs. All cell types show a response different from 0 (dorsal ipsilateral: P<1.0 × 10-8, dorsal contralateral: P=5.95 × 10-5, ventral: P<1.0 × 10-8) and all other cell types responded significantly less than dorsal ipsilateral cells (dorsal contralateral: P=1.06 × 10-3, ventral: P=7.22 × 10-3). |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |