- Title
-
A GFP-Tagged Gross Deletion on Chromosome 1 Causes Malignant Peripheral Nerve Sheath Tumors and Carcinomas in Zebrafish
- Authors
- Astone, M., Pizzi, M., Peron, M., Domenichini, A., Guzzardo, V., Töchterle, S., Tiso, N., Rugge, M., Meyer, D., Argenton, F., Vettori, A.
- Source
- Full text @ PLoS One
|
Phenotype of the ia2 mutant at 24 hpf. The homozygous mutant (C) displays a strongly delayed development and compromised phenotype, while the heterozygote (B) appears indistinguishable from the wild-type (A). The green fluorescence relative to the -8.5nkx2.2a:GFP transgene is present in heterozygous and homozygous ia2 mutant fish, being the GFP gene co-inherited with the ia2 mutant allele. This allows the early and immediate screening of the three genotypes. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Morphological alterations of ia2/ia2 mutant at 24 hpf. (A, A’) Head side view: mutant embryos display a relevant delay in head structures development, with just sketchy eye, when visible. (B, B’) Somites side view, showing their poorly defined shape and boundaries in the mutant. Globular cells with impaired adhesion are also visible. (C, C’) Notochord dorsal view: in mutant embryos notochord bending on the coronal plane is evident. (D, D’) Notochord side view, showing how notochord cells structure is also affected in mutant embryos, with globular and disorganized vacuoles, compared to the stretched and flanked ones of the wild-type. (E, E’) Tail terminal portion, side view: the notochord is indistinguishable in the mutant and the mesenchyme seems disaggregated with many non-adherent globular cells. Scale bar 50 μm. |
|
Incidence rate tumors in heterozygous ia2 mutant (ia2/+). (A) Cumulative percentages of tumor masses developed by ia2/+ individuals within 9, 12 and 16 months of age are depicted. (B) Representative images of the spontaneous tumor masses developed by heterozygous ia2/+ mutants, arising in the abdomen (top), along the spinal cord (middle) and intrathecally, causing the lateral projection of the eye (bottom). PHENOTYPE:
|
|
Representative histological features of the ia2 MPNSTs. (A) In normal controls (i.e. non-neoplastic cases), transverse sections of the abdominal cavity show the gastrointestinal tract (center), surrounded by pancreatic and adipose tissue. The liver (top center and lower left) occupies more peripheral regions. (B-E) Abdominal MPNST. (B) The tumor occupies the abdominal cavity under the swim bladder and infiltrates the intestinal wall (arrowheads). (C, D) Cytologically, the lesion is composed of spindle to epithelioid cells with high nuclear-to-cytoplasm ratio, clumped chromatin and evident mitoses (arrowhead in D). (E) The intra-abdominal MPNSTs stains negative for AE1/AE3 (positive internal control: intestinal epithelial cells, stained in brown). (F) In non-neoplastic cases, transverse sections of the cranial region display the oral cavity/pharynx (bottom center), lying beneath the basicranial structures (middle center) and the brain stem (top center). (G-L) Intrathecal MPNST. (G) The intrathecal neoplastic mass (star) compresses and displaces the adjacent medulla oblongata (arrowheads), without clear-cut infiltration of the neural parenchyma. (H, I) Cytologically, the lesion closely resembles its intra-abdominal counterpart. (L) Pan-cytokeratin immunostain is also negative in intrathecal MPNST (star). Arrowheads: tumor/brain stem interface. PHENOTYPE:
|
|
Representative histological features of the ia2 abdominal carcinomas. (A) Abdominal tumors arise beneath the swim bladder, in close proximity with the gut, pancreas and liver. The neoplastic cells occasionally infiltrate the hepatic parenchyma (arrows) and the intestinal wall (arrowhead). (B, C) Cytologically, the tumor is composed of polygonal cells with abundant eosinophilic cytoplasm. In most cases, a sub-population of epithelioid cells with more basophil cytoplasm is also faced (arrow). (D) The abdominal carcinomas consistently express the pan-cytokeratin AE1/AE3 (brown staining). See Fig 4A for the normal abdominal region. PHENOTYPE:
|
|
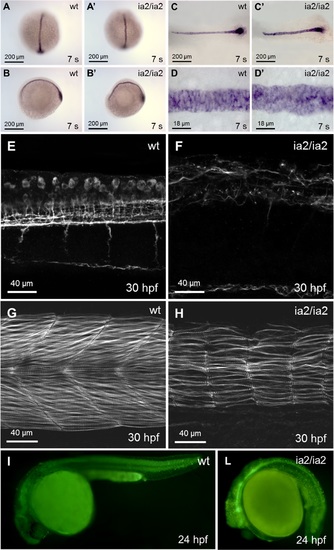
Phenotype of ia2/ia2 mutant. (A-D) Notochord alterations are evidenced by in situ hybridization for notail already at 7 somites stage. Mutant notochord is shorter and wider with respect to the wild-type (C, C’). Notochord bending (A-B’) and the morphological abnormalities of notochord structure and vacuolated cells (C-D’) are also apparent. (E, F) Axonal microtubules staining with anti-acetylated tubulin antibody. Confocal Z-stack projection; trunk side view. The mutant shows a highly disorganized axonal network. (G, H) Rhodamine-phalloidin staining. Confocal Z-stack projection; trunk side view. In the mutant the muscle fibers are strongly reduced in number, with an overall dramatic decline in muscle fibers mass. (I, L) Acridine orange staining. Massive cell death is evident in ia2/ia2 mutant, particularly in the regions of the head and the tail corresponding to the morphologically most altered districts. The intense GFP fluorescence of the neural tube visible in the mutant is that characteristic of the Tg(-8.5nkx2.2a:GFP)ia2 transgenic line. |