- Title
-
The miR-30 MicroRNA Family Targets smoothened to Regulate Hedgehog Signalling in Zebrafish Early Muscle Development
- Authors
- Ketley, A., Warren, A., Holmes, E., Gering, M., Aboobaker, A.A., and Brook, J.D.
- Source
- Full text @ PLoS One
|
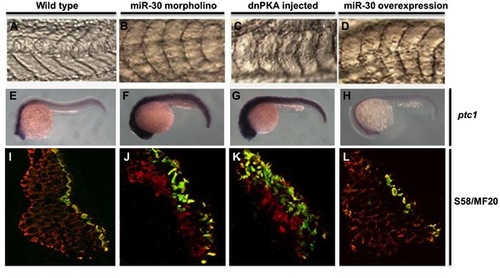
The miR-30 family is required during early embryonic development to regulate Hh pathway activity. Embryo somite structure at 24 hpf is shown (A–D). Ptc1 expression analysis was used as a read out of Hh pathway activity, showing elevated levels in miR-30 morpholino and dnPKA treated embryos when compared to wild type embryos (E–H). Slow muscle fibre number was quantified by immunohistochemistry using the S58 antibody (yellow/green) and MF20 staining (red). Embryo sections are orientated dorsal side upwards (I–L). Images are shown of wild type embryos (A, E, I), miR-30 morpholino treated embryos (B, F, J), dnPKA treated embryos (C, G, K) and miR-30 overexpression embryos (D, H, L). |
|
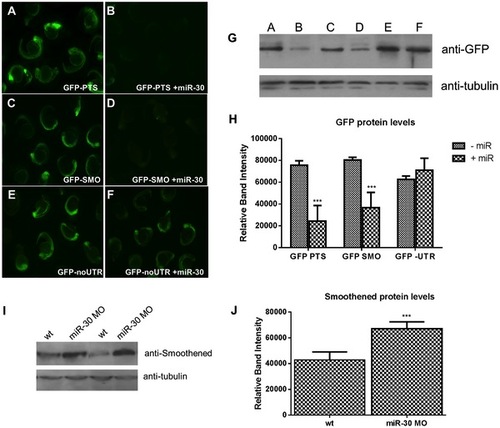
miR-30 directly targets the 3′UTR of the Hedgehog transmembrane receptor smoothened. (A–F) Embryos injected with 3 different GFP reporter mRNAs; (A,B) the GFP ORF plus tandem perfect target sites (GFP-PTS), (C,D) GFP ORF plus the smo 32UTR sequence (GFP-SMO) and (E,F) the GFP ORF without UTR sequence (GFP- no UTR). Constructs were injected either alone (A,C,E) or with the miR-30 duplex RNA (B,D,F). (G) Western blot validation on lysates of GFP injected embryos with and without the miR-30 duplex (H) Densitometric analysis of GFP protein levels normalised against α-tubulin loading control shows a 54% reduction in GFP-SMO+miR-30 compared to GFP-SMO only embryos. (I) Protein blot analysis of smoothened levels in wild type and miR-30 morpholino knockdown embryos shows an increased level of Smoothened protein. (J) Densitometric analysis of the average change in smoothened protein level in 3 samples of wild type versus miR-30 morpholino treated embryos. |
|
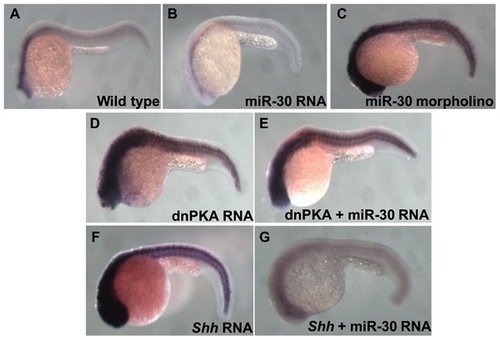
Analysis of Ptc1 reveals the position of miR-30 regulation in the Hh pathway. Ptc1 in situ hybridization shows the level of Hh pathway activity in different embryo treatment types. (A) Wild type embryo, (B) miR-30 overexpression embryo, (C) miR-30 morpholino injected embryo, (D) dnPKA overexpression embryo (E) dnPKA RNA injected with miR-30 duplex (F) Shh overexpression embryo (G) Reduced ptc1 expression is seen in Shh overexpression embryos coinjected with miR-30 RNA as miR-30 is able to suppress pathway activity. |
|
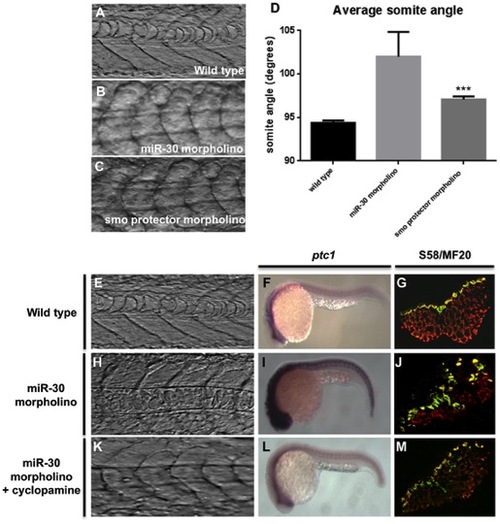
miR-30 acts to negatively regulate smoothened in developing embryos. (A–D) Somite angle analysis in wild type, miR-30 morpholino and smoothened protector morpholino injected embryos. Somite structure of (A) wild type embryos (B) miR-30 morpholino injected embryos (C) smoothened protector morpholino injected embryos. (D) Histogram to show the average somite angle in wild type and treated embryos. (E–M) Cyclopamine treatment causes reversion in somite structure to a more wild type phenotype. Images as shown of wild type embryos (E,F,G), miR-30 morpholino embryos (H,I,J) and miR-30 morpholino embryos treated with cyclopamine (K,L,M). The somite structure of (E) wild type (H) miR-30 morpholino and (K) miR-30 morpholino and cyclopamine treated embryos. (F, I, L) Expression of ptc1 is substantially reduced following cyclopamine treatment (L). Embryos are shown with anterior end to the left and dorsal side up. (G,J,M) The expanded band of slow fibres, as stained by S58 antibody, is restored to the wild type distribution following cyclopamine treatment (M). Embryo sections are orientated dorsal side to the left. |
|
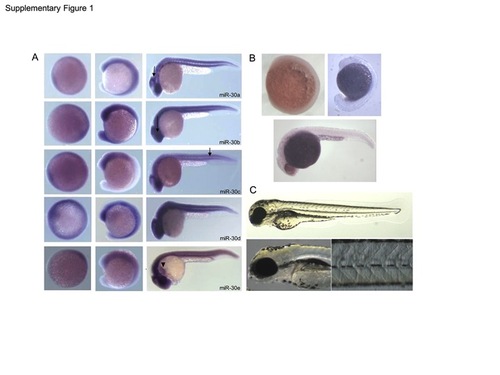
(A) Expression of the miR-30 family as determined by in situ hybridisation at 8, 16 and 26 hpf. Embryos are orientated anterior to the left and dorsal up. Expression is ubiquitous with predominant expression in the cerebellum, retina and somites, as indicated (arrows). miR-30e shows additional expression in the linear heart tube (arrowhead) (B) Negative control in situ hybridisation using a sense miR-159 LNA probe shows no detectable expression at 8 hpf, 16 hpf and 24 hpf. (C) Negative control morpholino against miR-140 showed no detectable phenotype when injected at the same concentration as the miR-30 morpholino upto 3 dpf. EXPRESSION / LABELING:
|
|
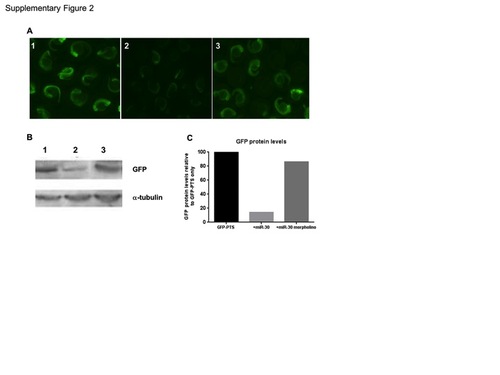
Validation of the miR-30 morpholino. (A) Injection of zebrafish embryos with GFP fused to a 3′UTR containing (1) tandem miR-30 perfect target sites (GFP-PTS). (2) Co-injection of miR-30 RNA with the GFP-PTS reporter mRNA. (3) Co-injection of miR-30 RNA and the miR-30 morpholino with the GFP-PTS reporter. (B) Western blot of embryos as in 1–3 with antibodies against GFP and α-tubulin as a loading control. (C) Histogram to quantify the restoration of GFP protein following miR-30 morpholino coinjection. GFP levels are normalised against α-tubulin and presented as a percentage of the GFP-PTS injected embryos. |
|
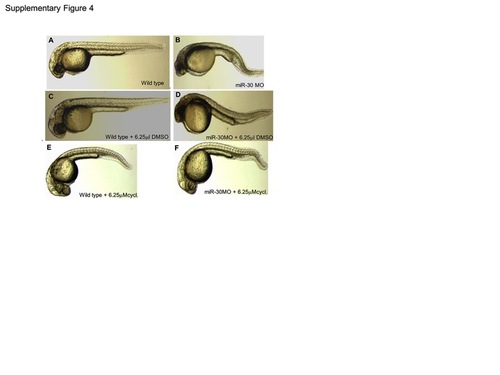
Cyclopamine treatment rescues the miR-30 morpholino phenotype. To achieve phenotypic rescue of the miR-30 morpholino phenotype cyclopamine was used at a concentration range of 100 μM-6.25 μM. At 6.25 μM the miR-30 morpholino phenotype improved to resemble the wild type phenotype with elongation of the tail and improved somite structure (F). Cyclopamine was dissolved in DMSO and both wild type and miR-30 morpholino injected embryos were treated with DMSO as a negative control (A–D) which had no effect on embryo development when compared to untreated. Wild type embryos treated with 6.25 μM cyclopamine showed a mild phenotype associated with Hh pathway inactivation with U shaped somites and a loss of brain chamber definition (E). |