- Title
-
Hemicentin 2 and Fibulin 1 are required for epidermal-dermal junction formation and fin mesenchymal cell migration during zebrafish development
- Authors
- Martins Feitosa, N., Zhang, J., Carney, T.J., Metzger, M., Korzh, V., Bloch, W., and Hammerschmidt, M.
- Source
- Full text @ Dev. Biol.
|
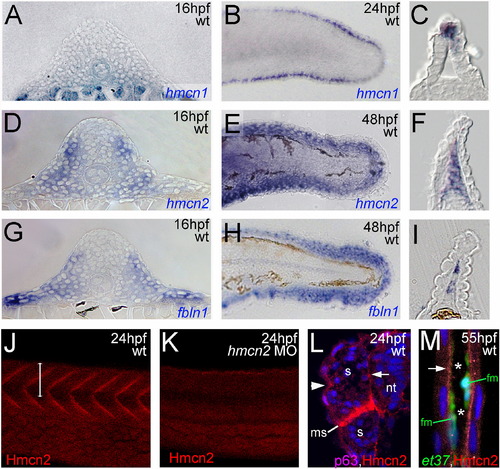
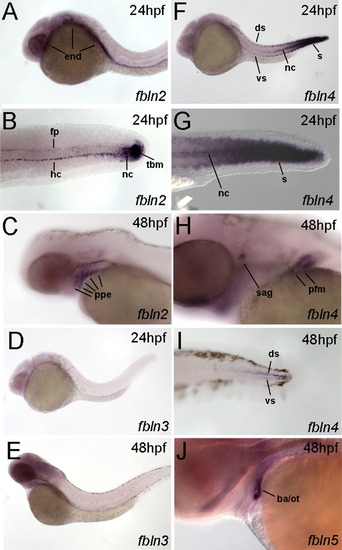
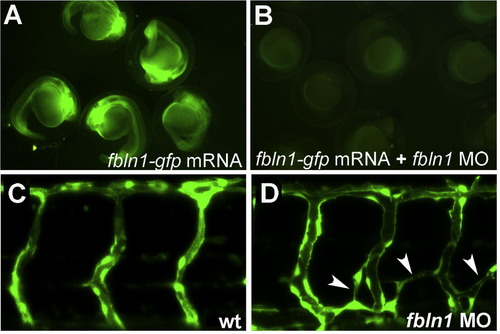
hmcn2 and fbln1 are co-expressed in somites and fin mesenchymal cells. (A–I) In situ hybridizations with probes indicated in lower right corners; (A, D, G) transverse sections through trunk at 26 hpf (14 somite stage); (B, E, H) lateral view on tail at 24 hpf (B) or 48 hpf (E, H); (C, F, I) transverse sections through tail of embryos as shown in (B, E, H), respectively; view on dorsal median fin fold. (A, D, G) hmcn2 (D) and fbln1 (G) are co-expressed in somites, whereas hmcn1 (A) is not. (B, C, E, F, H, I) hmcn1 (B, C) is expressed in epidermal cells at the apical ridge of the fin fold, while hmcn2 (E, F) and fbln1 (H, I) are expressed in fin mesenchymal cells within the dermal (interepidermal) space of the fin fold. (J–M) Anti-Hmcn2 immunostainings. (J,K) Lateral view on trunk at 24 hpf, revealing Hmcn2 protein at myosepta of uninjected control (J), whereas myoseptal staining is absent in hmcn2 morphant (K). (L) Transverse section through 24 hpf wild-type embryo at level indicated in (J). counterstained with anti-p63 antibody to stain nuclei of basal keratinocytes, and DAPI to visualize all nuclei. Arrowhead points to Hmcn2 protein in dermal space between epidermis and somites, arrow to Hmcn2 protein between neural tube and somites. (M) Transverse section through dorsal fin at 55 hpf embryo, showing Hmcn2 protein in red, mesenchymal cells in green (anti-GFP of ET37 transgene product), and DAPI-positive nuclei in blue. Hmcn2 protein is present in continuous lines below the epidermal sheets (blue nuclei). Strikingly, Hmcn2 protein is not restricted to regions that contain fin mesenchymal cells and their protrusions (in green), but also found in more apical regions (indicated by arrow), in line with its non-pericellular function during the guidance of mesenchymal cell migration described below (Fig. 4). However, inner regions of the dermal space of the fin lack Hmcn2 signals (indicated by stars). Abbreviations: wt, wild type; hmcn2 MO, hmcn2 morphant; hpf, hours post fertilization; ms, horizontal myoseptum; nt, neural tube; s, somite. EXPRESSION / LABELING:
|
|
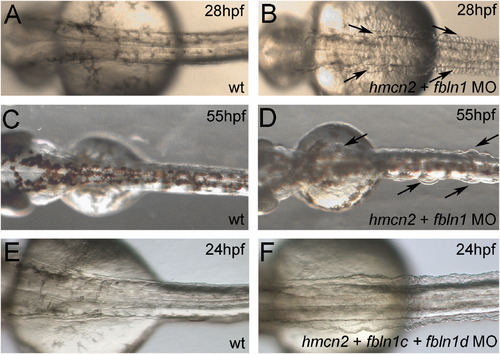
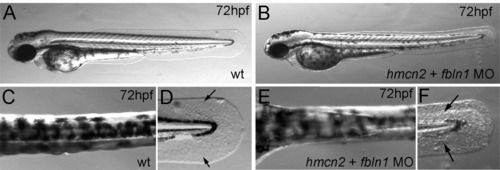
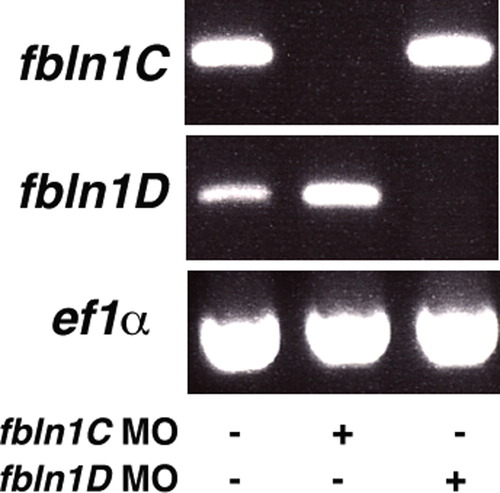
hmcn2/fbln1 double morphants display trunk blistering. Dorsal views on the trunk and tail of live embryos at stages indicated in upper right corners; anterior to the right. Arrows in embryos co-injected with hmcn2 MO and translation-blocking fbln1 MO in (B, D) point to blisters that are massive and widespread at 28 hpf (B), but alleviated and more locally restricted at 55 hpf (D). (E, F) Severe blistering is also obtained upon co-injection of hmcn2 MO with MOs targeting the two Fbln1 splice variants (fbln1c+fbln1d). |
|
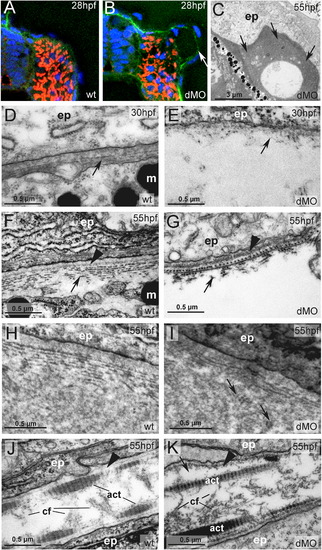
hmcn2/fbln1 double morphants display strongly compromised epidermal–dermal junction formation in the trunk, but not in the fin fold. (A, B) Transverse sections through trunk at 28 hpf, after anti-Laminin immunolabeling of BMs in green, Phalloidin-labeling of somitic muscle in red, and DAPI labeling of nuclei in blue. The double morphant (B) displays Laminin labeling at both sides of the blister, indicating that both epidermis and somites are properly attached to their respective basement membranes, and that blistering occurs within the dermal space in between. (C–K) Transmission electron micrographs of transverse sections through trunk (C–I) or dorsal tail fin (J, K) at stages indicated in upper right corners (for magnification, see scale bars). (C) Double morphant at low magnification, with most of trunk blister filled with amorphic material (indicated by arrows) at 55 hpf. (D, E) At 30 hpf, wild-type embryo (D) displays the first collagen fibers (arrow) underneath the yet indistinct BM, whereas much fewer fibers are present in the double morphant, with a fluid-filled blister underneath (E). Thinner and short fibers possibly integrated into and running perpendicular to the BM are visible in the double morphant (E; arrow), but more difficult to see in the wild-type (D, F), most likely due to the more compact organization of the tissue. It is tempting to speculate that they are the equivalent of the cross fibers of the fin fold (see Fig. 5), and involved in the organization of the ECM across the dermal space. (F, G) At 55 hpf, the collagenous network underneath the BM of the wild-type embryo consists of approximately ten layers organized in a plywood-like fashion (F; arrow), whereas the double morphant embryo displays a rupture within the second and third collagen layer (G; arrow). The shown specimen is one of the rare cases in which the blister seems to remain fluid-filled (see below). Arrowheads point to BMs. (H, I) In regions in which the architecture of the wild-type embryo requires a wider dermal space, such as at the base of the fins, it is filled with amorphic material underneath the sublaminal collagen fibers (H). In double morphants (I), most trunk blisters are filled with similar amorphic material, intermingled by disorganized and not properly anchored collagen fibers (arrows). (J, K) In the fin fold of wild-type embryos (J), collagenous actinotrichia are attached to the BM (indicated by arrowhead). In double morphant (K), actinotrichia and BM (indicated by arrowhead) appear unaffected, with rare and locally restricted detachments (indicated by arrow) in medio-basal regions of the fin. Abbreviations: act, actinotrichia; cf, cross fibers; dMO, double morphant; ep, epidermal cell; m, melanocyte. |
|
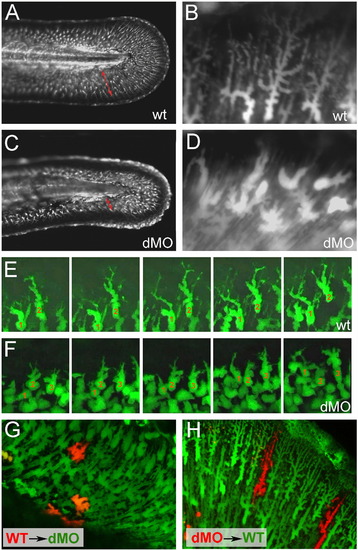
hmcn2/fbln1 double morphants display non-cell autonomous defects in fin mesenchymal cell migration. All panels show lateral views on the tail of live ET37 transgenic embryos, in which fin mesenchymal cells express GFP. (A–D) Overview (A, C) and magnified view of same embryos (B, D) at 55 hpf. Red arrows in (A, C) indicate the distance between the leading edge of fin mesenchymal cells and the base of the dorsal median fin, which is strongly reduced in the double morphant (C). Note that in the double morphant (C), the apical regions of the fin are collapsed, most likely as a secondary consequence of impaired mesenchymal cell immigration. Magnified views (B, D), showing that in wild-type embryo (B), fin mesenchymal cells have long protrusions that project laterally and apically (distally), whereas in the double morphant (D) cells have a more roundish shape with shorter and thicker protrusions. (E, F) Stills from time-lapse recordings of fin mesenchymal cells of wild-type (E) and double morphant embryo (F) from 35 to 40 hpf; individual cells are marked by numbers. (E) In wild-type, mesenchymal cells make stable protrusions that extend apically, followed by a displacement of the cell body into the same direction. (F) In the double morphant mesenchymal cells do form protrusions, however, protrusions are shorter and less persistent, and often (cell 2), but not always (cell 1), retract without cell body displacement. (G,H) Chimeric embryos at 72 hpf, generated via cell transplantations at early gastrula stage (6 hpf). (G) In double morphant host, wild-type mesenchymal cells (red) show compromised migratory behavior and cell shapes like their double morphant neighbors (green). (H) In wild-type host, double morphant mesenchymal cells (red) display highly arborized cells shapes like their wild-type neighbors (green), and can be found at the leading edge of the ingrowth, pointing to uncompromised migratory behavior. |
|
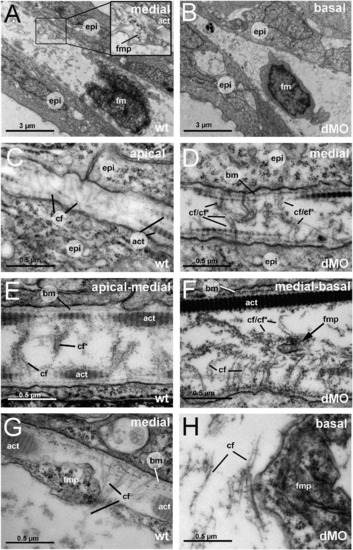
The fin folds of hmcn2/fbln1 double morphants display compromised cross fiber rearrangements and aberrant detachments of fin mesenchymal protrusions from collagenous actinotrichia. All panels show transmission electron micrographs of transverse sections through the dorsal median fin at 55 hpf. Positions of sections along the apical–basal (distal–proximal) axis of the fin are indicated in upper right corners. See scale bars for magnifications. (A, C, E, G) wild type; (B, D, F, H) hmcn2/fbln1 double morphant. (A) In wild-type embryo the mesenchymal cell is located in a medial region of the fin and has long projections that align along the actinotrichia (inset with magnification), projecting apically. (B) In double morphant, the space between the two epidermal sheets is very narrow, and the mesenchymal cell body is located in basal regions of the fin. (C) In apical-most regions of the wild-type fin, numerous cross fibers (cf) span the entire dermal space in parallel bundles and at right angles to the actinotrichia (act), connecting to the apposed epithelial sheets. (E) In apical-medial regions, but still apical of the fin mesenchymal cells and their protrusions, cross fibers display a bouquet-like organization in regions close to the cell surfaces and actinotrichia (cf), while in central regions of the dermal space, they are decorated by electron-dense material (cfN). (G) In medial regions, protrusions of fin mesenchymal cells are attached to the inner surface of the actinotrichia, while weaving between the cross fiber bouquets. (D) Medial fin regions of hmcn2/fbln1 double morphants (D) display an organization of cross fibers similar to that of apical-most regions of wild-type fins (compare with C), with cross fibers spanning the entire dermal space. (F) The few protrusion of fin mesenchymal cells that make it into basal-medial positions are not attached to the actinotrichia as in wild type, although actinotrichia are of normal thickness and pattern (compare with E), but found in central regions of the dermal space, surrounded by cross fibers. (H) Aberrant tight attachments between fin mesenchymal cells and cross fibers are also observed in the fin base of hmcn2/fbln1 double morphants. Abbreviations: act, actinotrichia; cf, cross fiber; cfN, decorated cross fiber; ep, epidermis; fm, fin mesenchymal cell; fmp, fin mesenchymal cell protrusion. PHENOTYPE:
|
|
|
|
|
|
|
|
|
Reprinted from Developmental Biology, 369(2), Martins Feitosa, N., Zhang, J., Carney, T.J., Metzger, M., Korzh, V., Bloch, W., and Hammerschmidt, M., Hemicentin 2 and Fibulin 1 are required for epidermal-dermal junction formation and fin mesenchymal cell migration during zebrafish development, 235-248, Copyright (2012) with permission from Elsevier. Full text @ Dev. Biol.