- Title
-
The epithelial cell adhesion molecule EpCAM is required for epithelial morphogenesis and integrity during zebrafish epiboly and skin development
- Authors
- Slanchev, K., Carney, T.J., Stemmler, M.P., Koschorz, B., Amsterdam, A., Schwarz, H., and Hammerschmidt, M.
- Source
- Full text @ PLoS Genet.
|
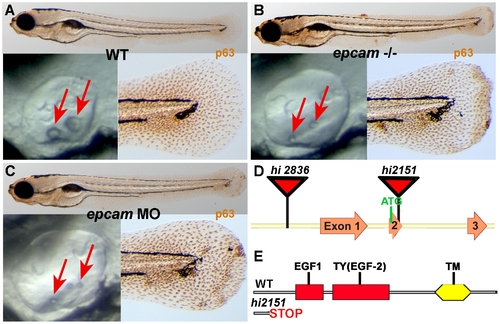
epcam mutants and morphants display smaller otoliths and keratinocyte aggregation. Panels (A–C) show wild-type siblings (WT, A), zygotic (M+/-,Z-/-) epcam mutants (epcam -/-) and epcam morphants (epcam MO); upper panels show larvae at 120 hpf after anti-p63 immunostainings of basal keratinocytes, lower right panels show magnification of tail tip regions, and lower left panels show lateral views on otic vesicles of live embryos at 48 hpf. Red arrows point to otoliths, which are smaller in mutants, but which recover later (see Figure S1). (D,E) Schematic illustration of the two epcam alleles generated by retroviral insertional mutagenesis [45]. In hi2836 the retroviral cassette is inserted upstream of exon1 (D), disrupting epcam transcription. In hi2151 the insertion is 42 base pairs downstream of the translational initiation site, introducing termination codons in all reading frames and leading to a premature termination of the protein that removes all described functional domains of EpCAM (EGF, epidermal growth factor-like domain; TY, thyroglobulin domain; TM, transmembrane domain) (D,E). PHENOTYPE:
|
|
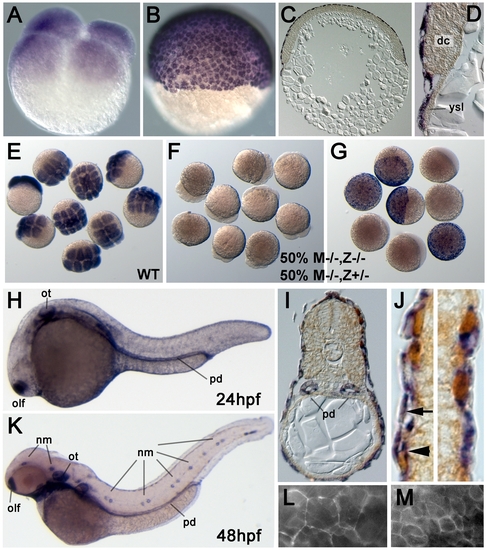
Zebrafish epcam is expressed in multiple epithelia, including the skin. (A–J) In situ hybridizations with anti-sense epcam probe. (A) Maternally provided epcam RNA is uniformly distributed in all blastomeres of 4-cell stage embryo (1 hpf). (B–D) At the 50% epiboly stage (early gastrula; 5.5 hpf), epcam expression is restricted to the outer EVL layer. (C,D) show 8 μm thick paraffin sections; in (D) epcam-negative deep cells (dc) and yolk syncitial layer (ysl) are indicated. (E–G) Mutant epcam mRNA is unstable, and zygotic epcam transcription is initiated before gastrulation. (E) Wild-type (WT) embryos at 8–16 cell stage; (F,G) embryos from cross of homozygous mutant female (M-/-) and heterozygous male, 50% of which are zygotic heterozygotes (Z+/-), and 50% zygotic homozygous mutants (Z-/-). At the 8–16 cell stage (F), all embryos lack (maternally supplied) epcam transcripts. At the 50% epiboly stage (G), Z-/- embryos still lack epcam mRNA, whereas zygotically derived transcripts are detectable in Z+/- embryos, indicating that zygotic epcam transcription starts shortly after midblastula transition [88]. (H–K) At 24 hpf (H–J) and 48 hpf (K), epcam is expressed in the olfactory placodes (olf), otic vesicles (ot), head and lateral line neuromasts (nm), pronephric ducts (pd) and the skin; (I,J) transverse sections through trunk of 24 hpf embryo labeled for epcam RNA (in blue) and p63 protein (in brown). epcam is expressed both in the (p63-negative) outer EVL (arrow in J), and in the underlying layer of p63-positive basal keratinocytes (arrowhead in J). (L, M) Epifluorescent images of live embryo at 9 hpf. Upon injection of mRNA encoding EpCAM-GFP, the fusion protein localizes to the cell membrane of both EVL cells (L) and the underlying deep cells (M). Note the size differences between the two cell types. |
|
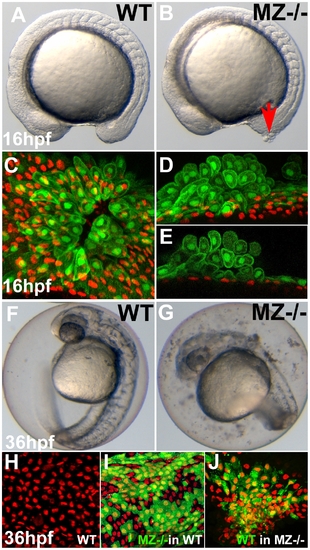
First cell aggregates of maternal/zygotic epcam mutants are formed during mid segmentation stages, starting in the EVL, whereas basal cell aggregates form secondarily. (A,B) Maternal/zygotic (MZ) epcam mutant at 16 hpf (12-somite stage) (B) with skin aggregate on tailbud (red arrow). (C–E) Confocal images of skin aggregate at 16 hpf; (C) merged stack of sagittal sections; (D) merged stack and (E) single layer of transverse sections; EVL cells labeled in green (anti-GFP immunostaining of tg(cytokeratin8:GFP) product), nuclei of basal keratinocytes in red (anti-p63 immunostaining). The EVL is ruptured (C), EVL cells have rounded up and have piled up on each other (D,E). In contrast, the underlying basal layer is normally organized, with regularly spaced p63-positive nuclei (D,E). (F,G) Live images of un-hatched embryos at 36 hpf. Sloughed skin cells are present within the chorion of mutant embryo (G). (H–J) Aggregates of basal keratinocytes are formed as a consequence of the loss of EpCAM function in the EVL; stacks of confocal images; transplanted basal cells are stained for GFP in green, nuclei of basal cells for p63 in red. (H) Non-chimeric wild-type control. (I) Chimeric embryo with cluster of MZepcam mutant basal cells (in green) in wild-type environment/underneath wild-type EVL, with normal spatial organization of mutant basal cells. (J) Chimeric embryo with cluster of wild-type basal cells (in green) underneath MZepcam mutant EVL, with aggregations of wild-type basal cells. PHENOTYPE:
|
|
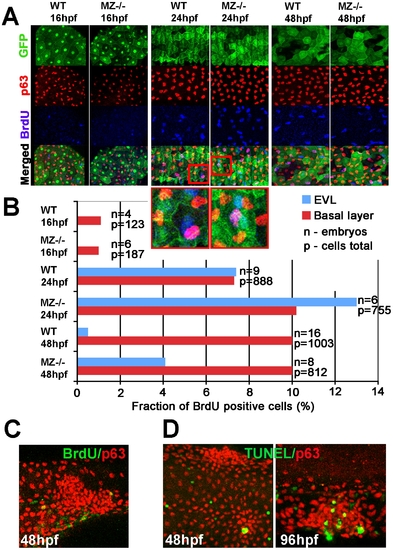
MZepcam mutants display secondary increase in proliferation and apoptosis of EVL cells and basal keratinocytes. (A) Confocal images of maternal/zygotic epcam (MZ-/-) mutant and wild type (WT) embryos at indicated stages. EVL cells and their nuclei are in green (anti-GFP immunostaining of tg(cytokeratin8:GFP) product; row 1), nuclei of basal keratinocytes in red (anti-p63 immunostaining; row 2), and BrdU-positive nuclei in blue (row 3). Row 4 shows merged images; row 5 magnified views of regions boxed in row 4 in red. They show examples of BrdU-positive EVL (in light blue; green+blue) and basal cell nuclei (in purple; red+blue). (B) Percentages of BrdU-positive EVL (in blue) and basal layer (in red) nuclei in WT and MZ-/- embryos at the indicated stages of development. (C) Confocal image of aggregate of p63-positive basal cells (in red) at 48 hpf; the aggregate is devoid of BrdU incorporation (in green), whereas BrdU-positive nuclei are present outside the aggregate, indicating that the assay worked. (D) Analysis of apoptosis levels within epidermal aggregates of MZ-/- embryos. At 48 hpf, aggregates contain very few or no TUNEL-positive cells (green), while more apoptotic cells are found in aggregates at 96 hpf. |
|
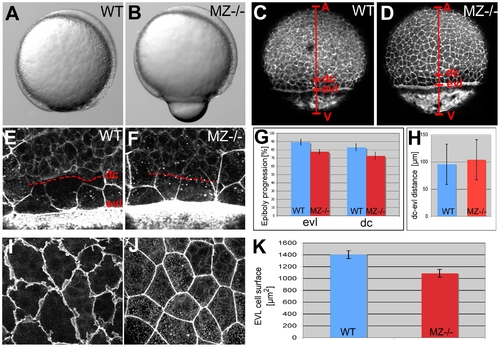
EpCAM is required for EVL and deep cell layer epiboly. (A,B) Live animals at the 90% epiboly stage, dorsal to the right; (C–F,I,J) phalloidin stainings of the actin cytoskeleton at 80% epiboly. (A,B) One of the morphogenetic processes during epiboly is the constriction of marginal EVL cells [10]. In mutants, this constriction does occur, however, at aberrant, slightly more animal positions, leading to a protrusion of the yolk plaque (B). (C,D) Overview over entire embryos; red bars indicate the animal (A) - vegetal (V) axis, and the margins of the deep cell layers (dc) and the enveloping layer (evl). The mutant (D) displays slightly retarded epiboly of the EVL and the deep cells (see larger distances between the evl margin and the vegetal pole (evl-V) and between the dc margin and the vegetal pole (d-V) compared to wild-type control in C). (G) Graphic demonstration of average progression of evl and dc epiboly in MZ-/- mutants and wild-type controls (WT). Percentage values were calculated from images like in (C,D) as (distance A-evl)/(distance A-V) or (distance A-dc)/distance (A-V). Standard deviations are indicated. For exact numbers see Text S1. (E,F) Magnified views on the evl and dc margins; dc margins are indicated by dashed red lines. (H) Graphic demonstration of average evl-dc distances in MZ-/- mutants and WT controls, as shown in (E,F). n = 5; standard errors are indicated. (I,J) Magnified views on EVL in regions slightly animal of the deep cell border (merged Z-stacks of confocal images), revealing ruffle-like protrusions in the wild type (I) that are missing in the mutant (J). The presence of intracellular, vesicular-like phalloidin staining varied from embryo to embryo, and even from cell to cell, but seemed unrelated to the epcam genotype. (K) Graphic demonstration of average horizontal sizes of EVL cells of WT and MZ-/- embryos at 80% epiboly, as shown in (E,F). n = 30; standard errors are indicated. PHENOTYPE:
|
|
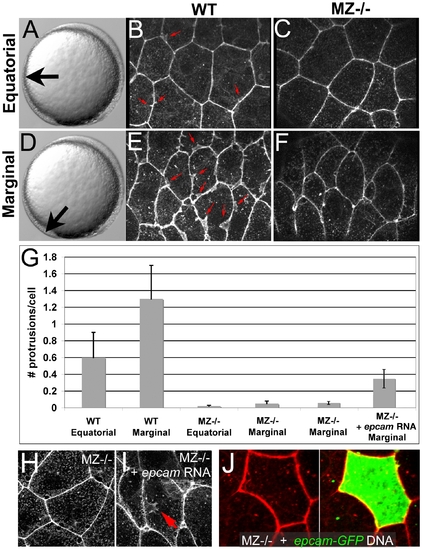
Ruffle formation in marginal EVL cells is partly restored upon uniform re-introduction of epam via RNA injection, whereas epcam overexpression in single EVL cells is insufficient. (A–F) Phalloidin stainings of wild type (WT) and maternal zygotic EpCAM mutant (MZ-/-) EVL cells at two different positions, equatorial (A–C) and marginal (D–F); merged Z-stacks of confocal images. In WT embryos, marginal EVL cells are much more protrusive than equatorial EVL cells, while in MZ-/- embryos, EVL cells show practically no protrusive activity at both locations. First four columns of panel (G) show quantification of protrusive activity. (H,I) Phalloidin stainings of marginal EVL cells from un-injected maternal/zygotic EpCAM mutant (MZ-/-) (H) and MZ-/- mutant injected with zebrafish epcam mRNA (I), 80% epiboly stage. There was a partial restoration of the protrusive activity of the EVL cells (red arrow in H), as quantified in (G), columns 5 and 6. Injection of higher amounts of epcam mRNA caused progressive death of injected embryos during pre- or early gastrula stages (data not shown), most likely due to ectopic effects of applied EpCAM in the deep cells, which precluded analyses of the EVL phenotype. (J) Injection of plasmid DNA encoding EpCAM-GFP fusion protein under the control of CMV promoter in MZ-/- embryos led to high recombinant protein levels in single cells. In most cases, this also caused death of expressing cells (data not shown). The few surviving EVL cells failed to restore ruffles (n = 0/13), suggesting that ruffle formation also requires EpCAM function in adjacent EVL cells, to which the protrusions usually attach (compare with Figure 7). Unfortunately, in over 100 investigated embryos, we failed to obtain clones with adjacent EpCAM-GFP-positive EVL cells, as would have been necessary to directly test this notion. PHENOTYPE:
|
|
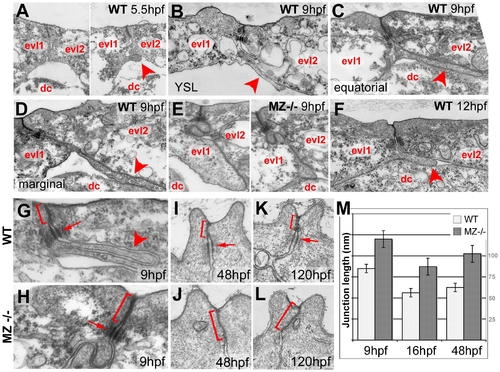
Basal protrusions and apical junctions are altered in MZepcam mutants. (A–L) Transmission electron micrographs of EVL–EVL interphase region of wild-type (A–D,F–K) and MZepcam mutant embryos (E,H–L) at indicated developmental stages. EVL and deep cells are indicated (evl1/2, dc; A–F), basal protrusions are marked by arrowheads (A–D,F,G), apical junctional complexes (AJCs) and their sizes by brackets (G–K), and desmosomes by arrows (G–I,K). (A–D) In wild-type embryos, basal protrusions start to form at early gastrula stages (A). Their lengths increase during gastrulation (B–D), and decrease after gastrulation is completed (F). Lengths in marginal EVL cells (D) are usually higher than in equatorially located EVL cells (C; see also Figure 6). Protrusions are also formed by marginal EVL cells spanning the cell-free zone between the deep cell layers and the yolk syncytial layer (YSL; [10]) (B). In mutants, basal protrusions are much shorter and broader (E). (G–L) AJCs are basally extended in the mutants (H,J,L), compared to wild-type siblings (G,I,K). Panel (M) shows graphical illustration of average lengths of AJCs in wild type and mutant cells at indicated developmental stages. n = 12; standard errors are indicated. PHENOTYPE:
|
|
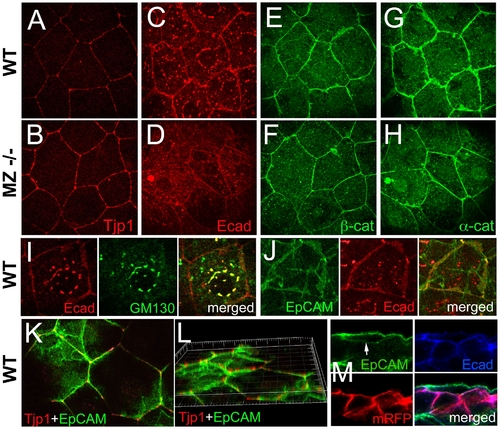
In wild-type EVL cells, EpCAM is localized at the basolateral membrane and excluded from the apical domain, while the molecular composition of apico-basal membranes is altered in MZepcam mutants. (A–H) Immunodetection of Tjp1 (A,B), Ecad (C,D), β-catenin (E,F) and α-catenin (G,H) in the EVL of wild-type (A,C,E,G) and MZepcam mutant embryos (B,D,F,H) at 90% epiboly stage. Images were acquired and processed using identical settings for mutant and wild-type samples. Mutants display increased membranous levels of the tight junction protein, but reduced levels of the cadherin-catenin complex. (I–M) Localization studies in wild-type EVL cells at 90% epiboly stage. (I) Double immunodetection of Ecad and the Golgi marker GM130-GFP [80], single and merged channels, revealing perinuclear E-cadherin in the Golgi apparatus. (J) Double immunodetection of Ecad and EpCAM-GFP, single and merged channels, revealing co-localization in a salt-and-pepper-like pattern (compare with [62]). (K,L) Double immunodetection of Tjp1 and EpCAM-GFP, revealing that EpCAM-GFP is localized basal to Tjp1; (L) shows rotated Z-stack. (M) Triple immunodetection of EpCAM-GFP, Ecad and RFP (labeling transplanted deep cells lacking EpCAM-GFP); single and merged channels. The white arrow indicates EpCAM-GFP localized in the basal membrane of the EVL cell (the underlying RFP-positive deep cells lack EpCAM-GFP). EXPRESSION / LABELING:
|
|
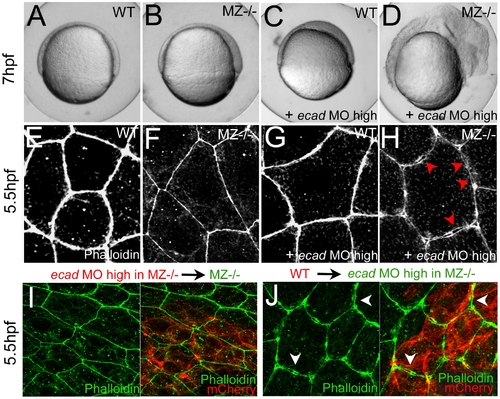
Combined complete loss of EpCAM and Ecad leads to compromised intercellular adhesion within the EVL of early gastrula embryos. (A–D) Live animals at the 70% epiboly stage (7 hpf); dorsal to the right; (E–J) phalloidin stainings of the actin cytoskeleton at 50% epiboly (5.5 hpf). (A,E) wild-type control (WT); (B,F,I) MZepcam mutant control; (C,G) WT injected with high amounts of E-cadherin morpholino oligonucleotide (ecad MO); (D,H,J) MZepcam mutant injected with high amounts of ecad MO. The regular ecad morphant (C) displays an arrest of deep cell epiboly at the equator, as previously described [10]–[12],[87], whereas EVL morphology appears normal (G). In contrast, EVL cells of the ecad;epcam double morphant/mutant embryo lose contact to each other (H; red arrowheads point to sites disassembly sites), and the embryo lyses (D). (I,J) The same EVL disassembly is observed in genetic mosaics, in which ecad;epcam double morphant/mutant EVL are positioned above transplanted wild-type deep cells (J; white arrows to disassembly sites), whereas wild-type EVL above ecad;epcam double morphant/mutant deep cells appear normal (I). Deep cells were transplanted at late blastula stages (3.5 hpf), and immunostained for the tracing marker mCherry in red. PHENOTYPE:
|
|
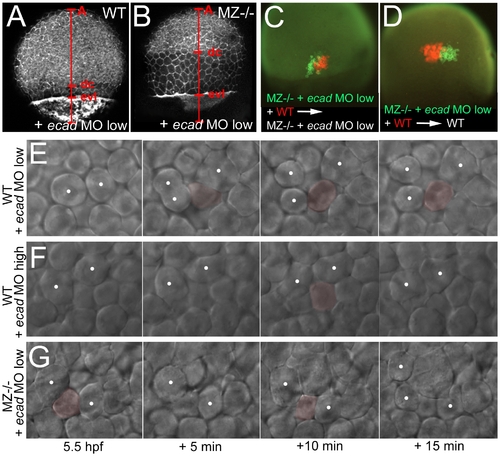
Concomitant partial loss of Ecad in the EVL of MZepcam mutants leads to severely compromised epiboly movements of deep cells. (A–D, E,G) wild-type (A,C,E) or MZepam mutant (B,D,G) embryos injected with low amounts of ecad MO; (F) wild-type embryo injected with high amounts of ecad MO. (A,B) Phalloidin stainings of the actin cytoskeleton at 80% epiboly stage (for explanation of labeling, see Figure 5). The ecad hypo-morphant appears normal (A; compare with Figure 5C as un-injected control), whereas partial loss of Ecad activity in the epcam mutants leads to an arrest of deep cell epiboly (B; note the reduced A-dc distance compared to panel A and to Figure 5D). In contrast, EVL epiboly is not more affected than in the un-injected mutant (B; note the normal A-evl distance compared to Figure 5D). (C,D) Genetic mosaics at 80% epiboly stage, after co-transplantation of deep cells from wild-type embryos (anti-RFP immunostaining; in red) and from MZepcam mutants injected with low amounts of ecad MO (anti-GFP immunostaining; in red), shortly before the onset of epiboly (4 hpf). Epiboly behavior of the transplanted deep cells does not depend on their own genotype, but on that of the host embryo/the overlying EVL. (E–G) Stills of time-lapse video recordings of the external layer of deep cells, starting at early gastrula stages (5.5 hpf). For evaluation, only cells were considered that moved up from internal into the external layer of the deep cells during the first half (0–10 min) of the recordings. Individual cells are pseudo-colored in light red when in the external layer, but un-colored when in lower layers. Note that the cell in (E) remains in the external layer throughout the second half of the video (10–20 min), whereas cells in (F,G) move up and down. White spots label representative neighboring cells of the external deep cell layer, demonstrating their increasing distance in (E), but constant distances in (F,G). PHENOTYPE:
|
|
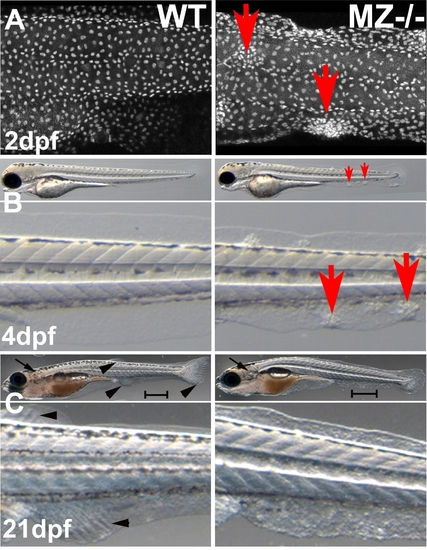
epcam mutants form skin cell aggregates. (A) Confocal image (merged Z-stack) of basal keratinocytes of wild type control (WT) and maternal/zygotic epcam mutant (MZ-/-) embryos at 2 days post fertilization (dpf), after anti-p63 immunostaining of basal keratinocytes. Basal cell aggregates of mutant are indicated by red arrows. (B,C) Overviews (upper panels) and magnified views of tail region (lower panels) of live WT (left panels) and MZ-/- mutants (right panels) at 4 days post fertilization (dpf) (B) and 21 dpf, when the skin becomes multi-layered (C). The epidermal aggregates of mutants persist during further larval development (B; indicated by red arrows), whereas they are less prominent at 21 dpf (C). In addition, otoliths have recovered and acquired normal size at 21 dpf (C; indicated by black arrows). However, the skin of the mutant has a rougher morphology, the mutant is of reduced size (C; see different lengths of scale bars = 1 mm), and fin development is delayed, as judged by the less advanced fin ray formation in the developing unpaired anal, dorsal and tail fins (C; indicated by black arrowheads in wild-type animal). Further analyses have to reveal whether this is a skin/fin-specific defect, or a consequence of generally delayed development, growth and metamorphosis. |
|
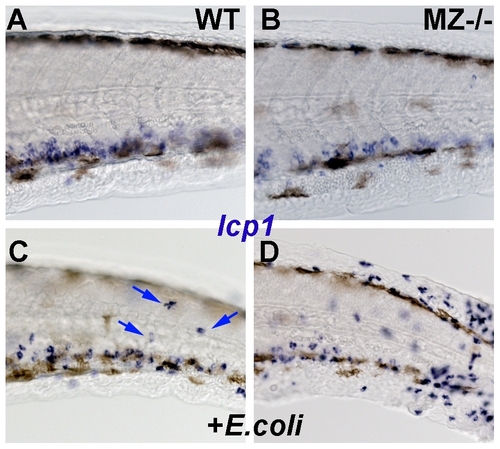
MZepcam mutants display increased susceptibility to cutaneous infections. All panels show lateral views on the tail region of embryos at 2 dpf, after whole mount in-situ hybridization for leukocyte-specific-plastin (lcp1) mRNA, a marker for leukocytes (macrophages and neutrophiles) [59]. (A,B) In wild-type (WT; A) and maternal-zygotic epcam mutants (MZ-/-; B) kept in semi sterile medium, leukocytes are found mainly in the blood vessels. (C,D) Addition of E. coli to the incubation medium stimulates the presence of innate immune cells in the skin (indicated by blue arrows in wild-type fish; C). Skin inflammation is much stronger in the challenged mutant (D) than in the wild-type control (C), suggesting that the mutant is more susceptible to cutaneous infections. |
|
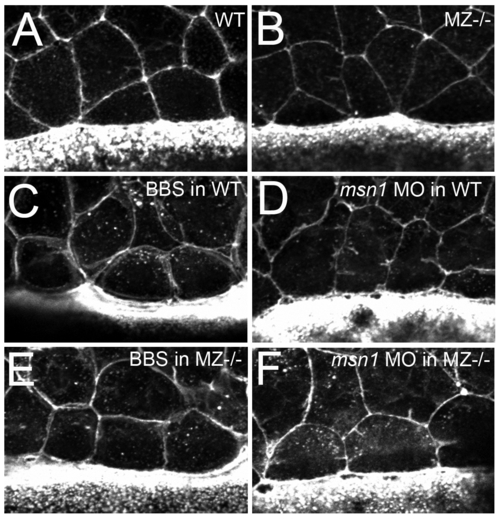
In contrast to loss of msn1 or treatment with blebbistatin, loss of epcam does not compromise the constriction of marginal EVL cells. (A–F) Fluorescent confocal images of phalloidin stained maternal zygotic-EpCAM mutant (MZ-/-) and wild type (WT) embryos at 70% epiboly. The leading EVL cells of both, mutant and WT embryos are constricted by an actin-myosin string as indicated by the presence of cells with shorter leading than trailing sides (A,B). This is in contrary to the release of constriction caused by in wild-type embryos upon treatment with 15 μg/ml blebbistatin (C) or injection of msn1 MO (D) [10], suggesting that the EVL epiboly defects of MZepcam and msn1 mutants have a different cellular basis, and that the phenotype of MZepcam mutants is not caused by reduced actin-myosin constriction at the EVL-YSL interface. Consistent with this notion, injection of msn1 MO or blebbistatin treatment in MZepcam mutants, while releasing marginal constrictions (E,F), failed to synergistically enhance the EVL epiboly defects, but rather had pure additive effects (data not shown). Another possibility would have been that the epiboly defects of MZepcam mutants are due to increased/precocious, rather than reduced actin-myosin constriction at the EVL-YSL interface. However, applying increasingly lower amounts of msn1 MO or blebbistatin to MZepcam mutants, we never obtained an alleviation of the EVL epiboly defects (judged by the extrusion of the vegetal-most part of the yolk at late gastrula stages; compare with Figure 5B), also making this possibility very unlikely. |
|
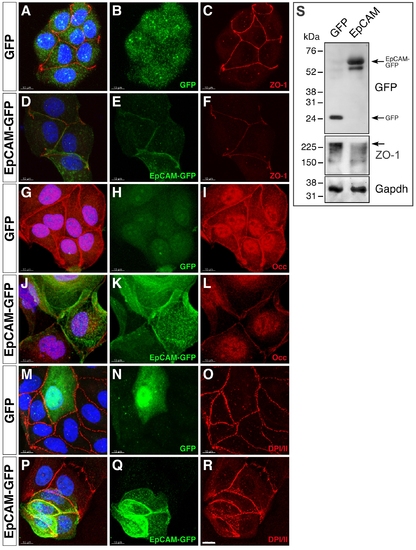
Overexpression of EpCAM in MDCK cells causes reduced levels of tight junction proteins. MDCK cells were transfected with expression vectors driving either GFP (A–C, G–I, M–O) or zebrafish EpCAM-GFP expression (D–F, J–L, P–R), plated on cover slips after FACS sorting one day after transfection, and stained for cell-cell junctional markers 48 hours after plating. Processed confocal images are shown as merges of entire Z-stacks. Transfectants were detected by GFP (B, H, N) or EpCAM-GFP expression (E, K, Q). Protein localization was analyzed with antibodies against Tjp1/ZO-1 (C, F), Occludin (Occ) (I, L), or Desmoplakin 1 and 2 (DPI/II) (O, R); panels (A,D,G,J,M,P) show overlays with GFP fluorescence. DAPI-stained nuclei are shown in blue (A,D,G,J,M,P); scale bar, 10 μm. Fluorescent labeling of key components of tight junctions reveals a reduction in membrane localization of Tjp1 in EpCAM- (F) versus GFP-transfected (C) cells. Similarly, Occludin staining is reduced in EpCAM expressing MDCK cells (I, L). However, proteins localizing to adherens junctions or desmosomes are not altered in expression or cellular distribution, as shown for DPI/II (O, R). This indicates that EpCAM expression in MDCK cells leads to a modification in apical junction complex assembly, resulting in a reduction of key tight junction proteins at the plasma membrane. (S) Immunoblot of lysats from FACS-sorted MDCK cells transfected with EpCAM-GFP (right lane) or, as control, GFP (left lane). EpCAM-GFP and GFP are expressed at comparable levels (upper panel). Expression of EpCAM-GFP leads to a significant in Tjp1/ZO1 protein levels (middle panel; arrow indicate full-length Tjp1/ZO1 protein). Gapdh was used as loading control (lower panel). |
|
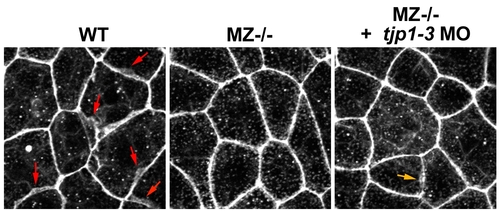
Knockdown of tight junction proteins 1–3 fails to restore ruffle formation in epcam mutants. Fluorescent confocal images of phalloidin stained wild type (WT) and maternal-zygotic EpCAM mutant (MZ-/-) embryos. Single and collective knock down of tight junction proteins 1–3 via antisense morpholino oligonucleotides (MO) injections into MZ-/- embryo (panel 2) failed to restore the protrusive activity of the mutant EVL cells back to the WT levels (panel 1, red arrows point to ruffles). If at all, rescue was very minor (orange arrow in panel 3 points to one of the few and rather small ruffles found in 12 investigated tjp1–3 MO-injected embryos). |
|
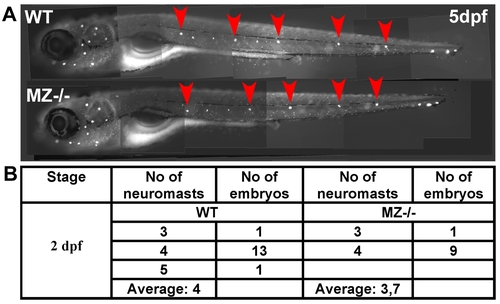
Maternal-zygotic epcam mutants have unaltered numbers of deposited primary neuromasts. (A) 5 dpf old maternal-zygotic epcam mutants (MZ-/-) and wild-type (WT) controls at 120 hpf, stained with 4-Di-2-ASP for vital labeling of differentiated hair cells in the larval lateral line. The red arrowheads point the deposited primary neuromasts. (B) Numbers of deposited primary neuromasts in WT control and MZ-/- mutants embryos at 2 dpf. Neuromasts were stained by their endogenous alkaline phosphatase activity [80]. Contrary to published data obtained from epcam morpholino studies [53], we could not detect significant differences in the numbers of deposited neuromasts between mutant and wild-type fish. |
|
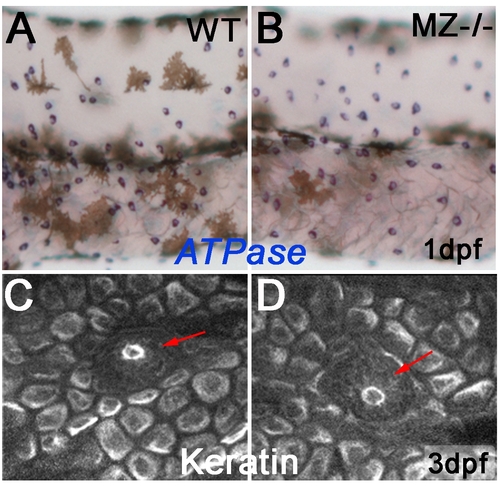
Keratinocytes and ionocytes of maternal-zygotic epcam mutants mutants express markers of terminal differentiation. To study whether EpCAM is required for terminal differentiation of epidermal cells, maternal/zygotic epcam mutants (MZ-/-; B,D) and wild-type controls (WT; A,C) were stained at 1 dpf for ATPase1b1b and ATPase6v1al transcripts, markers of two ionocytes subtypes, differentiated osmoregulatory skin cells that stem from the same pool of epidermal progenitors like keratinocytes (A,B), or at 3 dpf for keratin protein, a marker for differentiated basal keratinocytes of zebrafish larvae [74] (C,D). Mutants displayed normal signal intensities, normal numbers of ionocytes (A,B), and normal keratin distribution in basal cells (C,D), indicating that EpCAM is dispensable for the differentiation of epidermal cells. In addition, keratin distribution in primary neuromasts appeared normal (indicated by red arrows in C,D). |