PUBLICATION
Modulation of PPAR signaling disrupts pancreas development in the zebrafish, Danio rerio
- Authors
- Venezia, O., Islam, S., Cho, C., Timme-Laragy, A.R., Sant, K.E.
- ID
- ZDB-PUB-210728-18
- Date
- 2021
- Source
- Toxicology and applied pharmacology 426: 115653 (Journal)
- Registered Authors
- Keywords
- Development, Organogenesis, PPAR, Pancreas, Peroxisome proliferator-activated receptor, Zebrafish, β Cells
- MeSH Terms
-
- Pancreas/abnormalities*
- Abnormalities, Multiple
- Female
- Embryonic Development
- Animals, Genetically Modified
- PubMed
- 34302850 Full text @ Tox. App. Pharmacol.
- CTD
- 34302850
Abstract
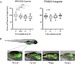
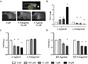
Peroxisome Proliferator Activated Receptors (PPARs) are transcription factors that regulate processes such as lipid and glucose metabolism. Synthetic PPAR ligands, designed as therapeutics for metabolic disease, provide a tool to assess the relationship between PPAR activity and pancreas development in vivo, an area that remains poorly characterized. Here, we aim to assess the effects of PPAR agonists and antagonists on gene expression, embryonic morphology and pancreas development in transgenic zebrafish embryos. To evaluate developmental perturbations, we assessed gross body and pancreas morphology at 4 days post fertilization (dpf) in response to developmental exposures with PPARα, PPARγ, and PPARβ/δ agonists and antagonists at 0, 0.01, 0.1, 1, and 10 μM concentrations. All ligand exposures, with the exception of the PPARα agonist, resulted in significantly altered fish length and yolk sac area. PPARγ agonist and antagonist had higher incidence of darkened yolk sac and craniofacial deformities, whereas PPARα antagonist had higher incidence of pericardial edema and death. Significantly reduced endocrine pancreas area was observed in both PPARγ ligands and PPARα agonist exposed embryos, some of which also exhibited aberrant endocrine pancreas morphology. Both PPARβ/δ ligands caused reduced exocrine pancreas length and novel aberrant phenotype, and disrupted gene expression of pancreatic targets pdx1, gcga, and try. Lipid staining was performed at 8 dpf and revealed altered lipid accumulation consistent with isoform function. These data indicate chronic exposure to synthetic ligands may induce morphological and pancreatic defects in zebrafish embryos.
Genes / Markers
Expression
Phenotype
Mutations / Transgenics
Human Disease / Model
Sequence Targeting Reagents
Fish
Orthology
Engineered Foreign Genes
Mapping