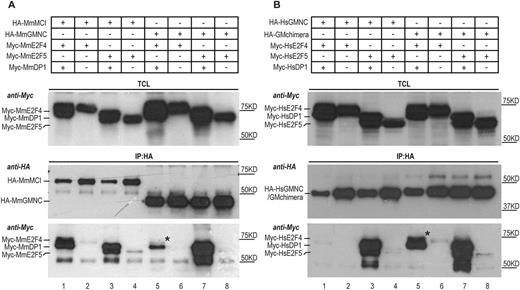
Fig. 7 Differential interaction of MCI and GMNC with E2F4 and E2F5. (A) Co-immunoprecipitation experiments using mouse (Mm, Mus musculus) proteins show that MCI effectively interacted with both E2F4 and E2F5 in the presence of DP1 (lane 1, IP panel, anti-Myc blot); very little E2F4 co-precipitated with GMNC and DP1 (lane 5, IP panel, anti-Myc blot). The weak presence of E2F4 in the GMNC IP is marked by the black asterisk. (B) The C-terminal domain of MCI accounts for effective interaction with both E2F4 and E2F5. GMNC showed minimal interaction with E2F4 (lane 1, IP panel, anti-Myc blot). In comparison, the GM (engineered by replacing the C-terminal domain of GMNC with that of MCI) chimera could co-precipitate with both E2F4 and E2F5, in the presence of DP1 (lane 5 and 7, IP panel, anti-Myc blot). The black asterisk marks the E2F4 band that is absent in lane 1 (IP panel, anti-Myc blot). Human (Hs, Homo sapiens) proteins were used for this experiment. The E2F and DP1 proteins were tagged N terminally with the Myc epitope, and the GMNC and MCI proteins were tagged N terminally with the HA epitope. TCL, total cell lysate; IP, immunoprecipitation. Data are representative of two independent biological replicates.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Development