Fig. S3
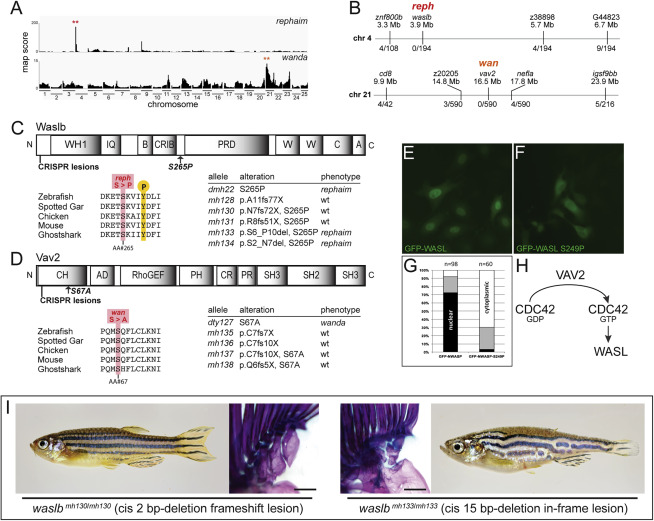
Figure S3. Mutations in waslb and vav2 cause the reph and wan phenotypes, respectively, related to Figure 1 (A) Mapping score from homogeneity-by-descent analysis for reph and wan mutants based on whole-exome sequencing of homozygous populations. Asterisks highlight regions of putative linkage. (B) Fine mapping of the chromosome with the highest mapping score by recombination analysis in individual homozygous mutants. Shown are informative markers used, their position, and the number of recombinants identified per meiosis scored. Within the smaller linked interval, sequence data from the mutant pool was used to identify nonsynonymous SNPs as candidate mutations responsible for the altered skeletal phenotype of reph and wan. (C, D) Protein schematics of Waslb (C) and Vav2 (D) showing positions of missense mutations and CRISPR-generated lesions. Multiple sequence alignment reveals conservation of the mutated residues across vertebrates. Mutant alleles are listed with their consequence on amino acid sequence and their phenotypic effect. (E-G) HeLa cells transfected with constructs encoding N-terminal GFP fusions to mouse wild-type WASL (GFP-WASL, E) and an WASL version containing the orthologous reph mutation S249P (GFP-WASL, F). (G) Qualitative scoring of transfected cells; white, predominantly cytoplasmic localization; black, predominantly nuclear localization; gray, no bias observed in localization. (H) Summary of known protein-protein interactions that link Wasl and Vav2 proteins. Vav2 acts as a guanine nucleotide exchange factor for Cdc42, converting Cdc42-GDP to Cdc42-GTP. GTP-bound Cdc42 is a regulator of Wasl activity. (I) Phenotype of cis frameshift and cis in-frame lesions upstream of the reph mutation. Scale bars 2 mm.
Reprinted from Cell, 184(4), Hawkins, M.B., Henke, K., Harris, M.P., Latent developmental potential to form limb-like skeletal structures in zebrafish, 899-911.e13, Copyright (2021) with permission from Elsevier. Full text @ Cell