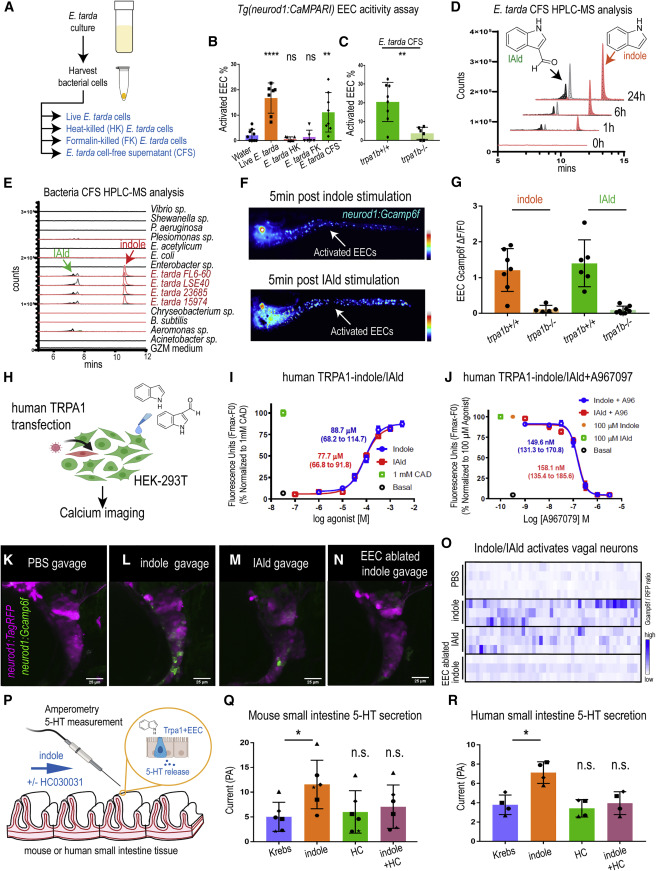
Fig. 7 ure 7. E. tarda derived tryptophan catabolites activate Trpa1 and the EEC-vagal pathway (A) Method for preparing different fractions from E. tarda GZM (zebrafish water) culture. (B) Activated EECs in Tg(neurod1:CaMPARI) zebrafish stimulated by different E. tarda fractions. (C) Activated EECs in trpa1b+/+ and trpa1b−/− Tg(neurod1:CaMPARI) zebrafish stimulated with E. tarda CFS. (D) Screening of supernatants of E. tarda in GZM culture medium by HPLC-MS. Samples were collected at 0, 1, 6, and 24 h. Abbreviations are as follows: IAld, indole-3-carboxaldehyde; and IEt, tryptophol. Extracted ions were selected for IAld (m/z 145), IEt, (m/z 161), and indole (m/z 117). (E) Chemical profiles of Trp-indole derivatives from supernatants of various commensal bacteria in GZM medium for 1 day of cultivation. y axis values represent the production of Trp-indole derivatives normalized to CFU, with each strain beginning at zero. (F) Tg(neurod1:Gcamp6f) zebrafish stimulated by indole or IAld. Activated EECs in the intestine are labeled with white arrows. (G) Quantification of EEC Gcamp activity in trpa1b+/+ and trpa1b−/− zebrafish stimulated with indole or IAld. (H) Schematic of experimental design to test the effects of indole and IAld on human or mouse Trpa1. (I) Dose-response analysis of the integrated Calcium 6 fluorescence response above baseline (Fmax.-F0; maximal change in Ca2+ influx) as a function of indole and IAld concentration in human TRPA1 expressing HEK-293T cells. (EC50 = 88.7 μM, 68.2–114.7 μM 95% CI for indole; and, EC50 = 77.7 μM, 66.8–91.8 μM 95% CI for IAld) Concentration-response data were normalized to 1 mM CAD, a known TRPA1 agonist. Data represent the mean of 3–4 experiments, each performed with 3–4 replicates. (J) Dose-response analysis of A967079 inhibition of indole and IAld-induced Ca2+ influx. (IC50 = 149.6 nM, 131.3–170.8 nM 95% CI for indole; and, IC50 = 158.1 nM, 135.4–185.6 μM 95% CI for IAld) Concentration-response data of A967079 inhibition were normalized to response elicited by 100-μM agonist (indole or IAld). (K–N) In vivo calcium imaging of vagal sensory ganglia in WT (K-M) or EEC-ablated (N) Tg(neurod1:Gcamp6f); Tg(neurod1:TagRFP) zebrafish gavaged with PBS (K), indole (L,N) or IAld (M). (O) Quantification of individual vagal sensory ganglia cell Gcamp6f fluorescence intensities in WT or EEC-ablated zebrafish gavaged with PBS or 1 mM indole. (P) Schematic of amperometric measurements to examine the effects of indole on 5-HT secretion in mouse and human small intestinal tissue. (Q) Indole caused a significant increase in 5-HT secretion in mouse duodenum; however, no such effects were observed in the presence of Trpa1 antagonist HC030031. (R) Indole caused a significant increase in 5-HT secretion in human ileum; however, no such effects were observed in the presence of Trpa1 antagonist HC030031. Data in (B, C, G, Q, and R) are presented as mean ±SD. One-way ANOVA with Tukey’s post-test was used in (B and Q), Student’s t test was used in (C and H) and paired one-way ANOVA with Tukey’s post-test was used in (P–R). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Cell Host Microbe