Fig. 8
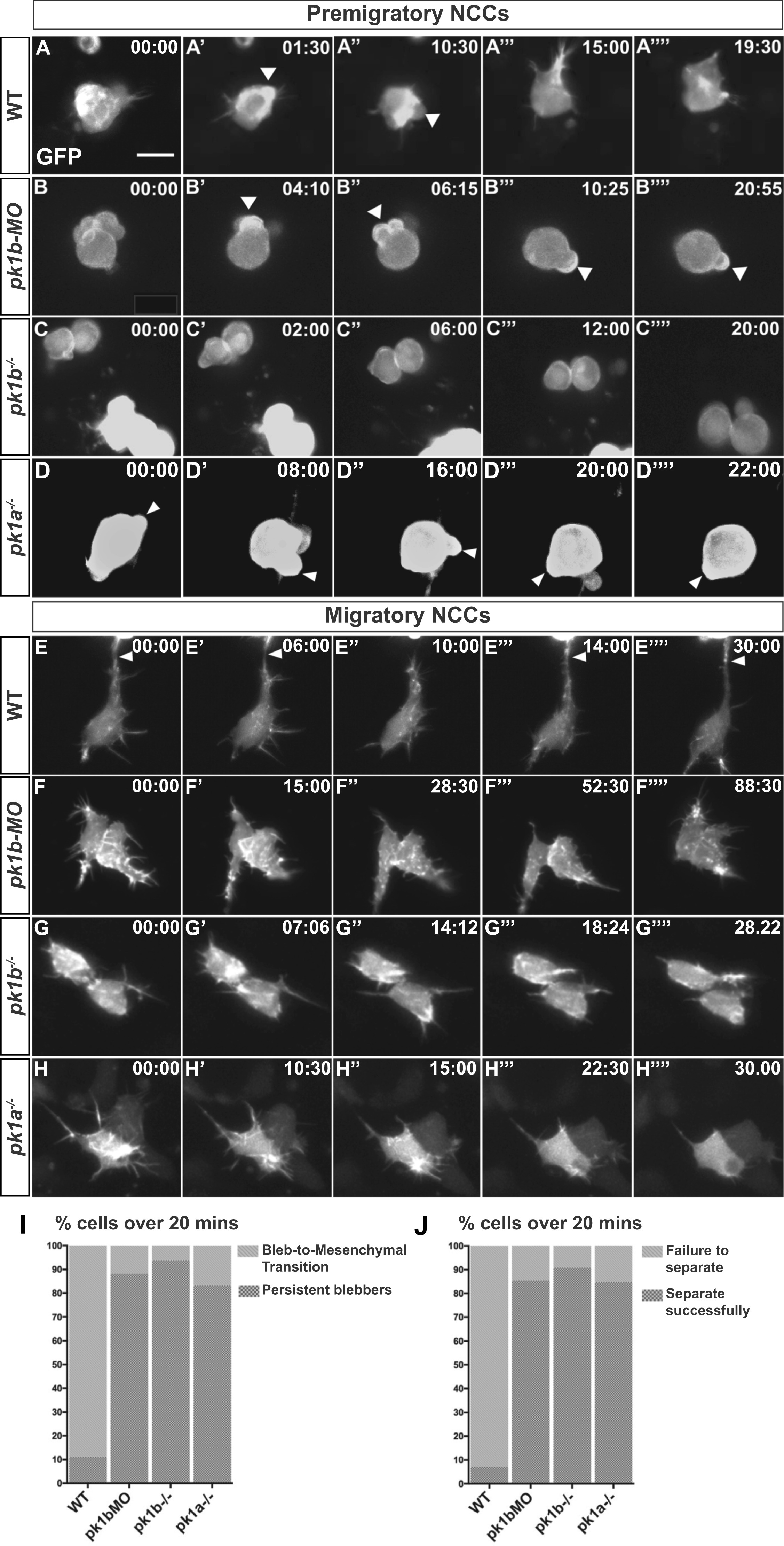
Loss of Pk1 function causes defects in both pre-migratory NCCs in the transitional states of EMT, as well as in migratory ‘escaper’ NCCs. To query the role of Pk1 molecules in the morphological transitions of EMT, as well as during active migration, NCCs were labeled in a mosaic fashion by injecting DNA encoding LifeAct-GFP under the control of a sox10 promoter into one-cell stage embryos. (A-H) Confocal time-lapse imaging of LifeAct-GFP positive cells for at least 20 min in 16 hpf embryos revealed F-actin rich protrusions and distinct morphologies of NCCs. Scale bar= 10 µm. (A-D′′′′) Frames from confocal time-lapses of pre-migratory NCCs. (A-A′′′′) WT NCCs display short protrusions even before displaying rounded bleb-based protrusions. WT NCCs adopt the bleb-based morphology before transitioning to morphologies with longer filopodial protrusions, with NCCs moving through these transitional morphologies in EMT over the time-span of approximately 20 min. (B-B′′′′, C-C′′′′, D-D′′′′) Over the same time frame as WT NCCs, pk1b-morphant, pk1b fh122/ fh122, and pk1ach105/ch105 NCCs show bleb-based protrusions that are actively maintained, on occasion changing the location of the bleb along the edges of a NCC. (B′-B′′′) White arrowheads indicate bleb-based protrusions. (E-H) Confocal time-lapse images of NCCs in the mandibular stream actively migrating towards the first pharyngeal arch. (E-E′′′′) Actively-migrating WT cells that are highly filopodial make transient contacts with neighboring cells, often making, breaking and re-establishing a contact in the form of a thick actin-rich protrusion with the same neighboring cell over a time span of at least 20 min. White arrowheads indicate a protrusion contacting a neighboring NCC. (F-F′′′′, G-G′′′′, H-H′′′′) pk1b-morphant, pk1b fh122/ fh122, and pk1ach105/ch105 migratory NCCs are highly protrusive but display an inability to separate from neighboring NCCs and maintain contact, often over extended time periods. (I) Bar graph showing the percentage of pre-migratory NCCs that successfully transitioned from a bleb-based to a highly-protrusive mesenchymal-like morphology relative to those that remained in the persistently-blebbing state over the time span of 20 min. 89% of WT NCCs (n = 18 cells, 8 embryos), 16% of pk1b-morphant NCCs (n = 17 cells, 8 embryos), 6% of pk1b fh122/ fh122 NCCs (n = 32, 8 embryos), and 17% of pk1ach105/ch105 NCCs (n = 12 cells, 5 embryos) transitioned successfully. (J) Bar graph showing the percentage of migratory NCCs that established and severed contact with a neighboring NCC relative to NCCs that failed to sever contact with a neighboring NCC over the time span of 20 min. 93% of WT NCCs (n = 28, 6 embryos) NCCs, 15% of pk1b-morphant NCCs (n = 41 cells, 10 embryos), 9% of pk1b fh122/ fh122 NCCs (n = 22 cells, 7 embryos), and 15% of pk1ach105/ch105 NCCs (n = 13 cells, 4 embryos) severed contact with neighboring NCCs.
Reprinted from Developmental Biology, 448(1), Ahsan, K., Singh, N., Rocha, M., Huang, C., Prince, V.E., Prickle1 is required for EMT and migration of zebrafish cranial neural crest, 16-35, Copyright (2019) with permission from Elsevier. Full text @ Dev. Biol.