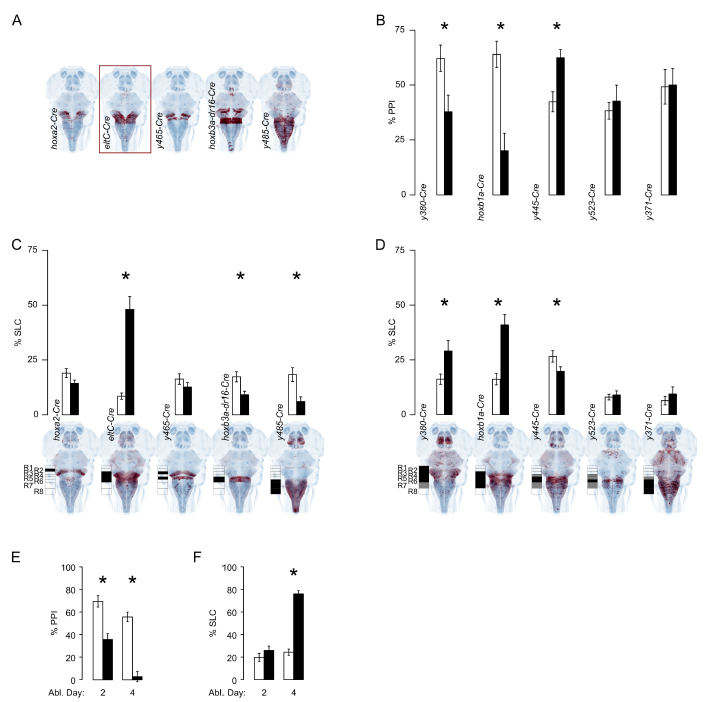
Fig. S3
Intersectional genetic ablations and developmentally restricted ablations parse the Gsx1 population to reveal subsets that regulate acoustic startle. Related to Figure 4.
A. Horizontal projection of the genetic intersect pattern (red) of Gsx1 (gsx1-Gal4) and a Cre line registered to ZBB (HuC, blue). Border indicates intersection ablation that decreased prepulse inhibition of startle.
B. Prepulse inhibition (% PPI) of acoustic startle in larvae with rhombomere-specific ablation of Gsx1 neurons (black), or metronidazole-treated clutchmates without nitroreductase expression (white). WRS test, number of fish (control, ablated): y380-Cre P=0.03 (17, 14), hoxb1a-Cre P=0.0002 (23,25), y445-Cre P=0.002 (40,35), y523-Cre P=0.5 (34,19), y371-Cre P= 0.96 (23,18).
C. Startle responsiveness (% SLC) to a prepulse alone in larvae with rhombomere-specific ablation of Gsx1 neurons (black), or metronidazole-treated clutchmates without nitroreductase expression (white). Prepulse inhibition (% PPI) of startle in the same transgenic lines in Figure 4C. Unpaired t-test, number of larvae (control, ablated): hoxa2-Cre P=0.06 (133, 149), eltC-Cre P=3x10-14 (77, 34), y465-Cre P=0.3 (69, 56), hoxb3a-dr16-Cre P=0.004 (102, 102), y485-Cre P=0.001 (35, 43). C,D: Projections show the full pattern of Cre expression with a schematic indicating rhombomere expression in each line.
D. SLC to a prepulse alone for larvae with rhombomere-specific ablation of Gsx1 neurons (black), or metronidazole-treated clutchmates without nitroreductase expression (white). Unpaired t-test, number of fish (control, ablated): y380-Cre P=0.01 (33,28), hoxb1a-Cre P=0.00002 (51,51), y445-Cre P=0.04 (64,71), y523-Cre P=0.6 (54,35), y371-Cre P=0.4 (34,34). *P<0.05. Increased responsiveness was only seen when R4 Gsx1 neurons were ablated, indicating that these neurons negatively regulate startle responsiveness. Conversely, ablation using 3 different Cre lines (hoxb3a-dr16-Cre, y485-Cre and y445-Cre) that comprise R6 Gsx1 neurons reduced startle responsiveness, uncovering an unexpected role for these neurons in maintaining normal acoustic startle sensitivity that is masked by ablation of all Gsx1 neurons.
E-F. Earlier-generated Gsx1 neurons are required for prepulse inhibition but not startle threshold regulation. Prepulse inhibition (% PPI, E) and startle responsiveness (% SLC, F) after a 1-day metronidazole treatment starting on the indicated day to ablate Gsx1 cells in nitroreductaseexpressing y252-Gal4, UAS:epNTR larvae (black) and non-transgenic clutchmates (white). Twosided unpaired t-test, number of fish (control, ablated): day 2 treatment, PPI: P=0.03 (28, 33), SLC: P=0.2 (42, 37), day 4 treatment, PPI: P=3x10-12 (51, 31), SLC: P=7x10-26 (72, 72). *P<0.05. All error bars are s.e.m. Thus, ablation of all Gsx1 neurons generated before 5 dpf both disrupted prepulse inhibition and reduced acoustic startle thresholds. In contrast, prepulse inhibition was selectively impaired by ablation of Gsx1 neurons generated before, but not after, 3 dpf. Distinct Gsx1 neurons thus regulate prepulse inhibition and startle thresholds, despite both of these groups being present in R4.