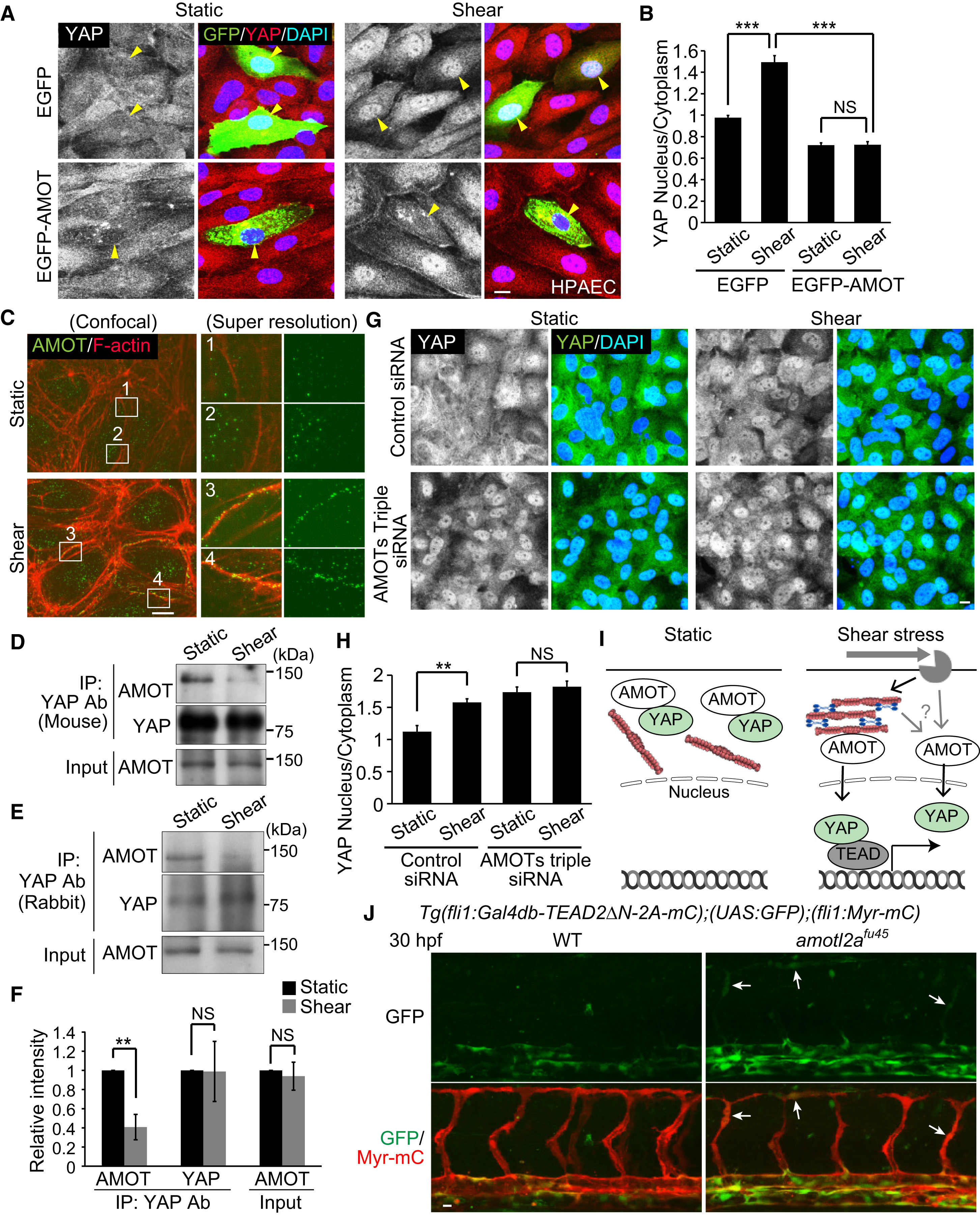
Fig. 6
Angiomotins Are Involved in the Regulation of Nuclear Translocation of YAP Induced by Shear Stress
(A) HPAECs transfected with EGFP or EGFP-AMOT were kept under static conditions or subjected to shear stress (15 dynes/cm2, 10 min) and immunostained with anti-YAP and anti-GFP antibodies together with DAPI. YAP images (left) and the merged images (right; EGFP, green; YAP, red; DAPI, blue) are shown. Yellow arrowheads indicate EGFP-expressing cells.
(B) Quantification of nuclear fluorescence intensity of YAP relative to that of cytoplasm in EGFP- or EGFP-AMOT-expressing HPAECs as in (A) (n = 3 independent experiments, in each of which >20 EGFP-expressing cells measured).
(C) HPAECs under static conditions or after shear stress were immunostained with anti-AMOT antibody and stained with rhodamine-phalloidin (F-actin). Super-resolution images (right) were acquired in the boxed regions of confocal fluorescence images (left). In the right panels, the merged images (left; AMOT, green; F-actin, red) and AMOT images (right: green) are shown. Representative images of two independent experiments are shown.
(D and E) Lysates of static or sheared HPAECs were immunoprecipitated with mouse anti-YAP (D) or rabbit anti-YAP antibody (E). Coprecipitated endogenous AMOT (p130) was detected by immunoblotting with anti-AMOT antibody. Similar results were obtained in three independent experiments using each antibody.
(F) Total AMOT (Input) and the AMOT and YAP immunoprecipitated with rabbit anti-YAP antibody (IP: YAP Ab) in static or sheared HPAECs as in (E) were analyzed. The relative intensity was calculated by the intensity of the band of immunoprecipitated AMOT or YAP by anti-YAP antibody in the static or sheared HPAECs (n = 3 independent experiments).
(G) HPAECs transfected with control siRNA or AMOT + AMOTL1 + AMOTL2 siRNAs (AMOTs triple siRNAs) were kept under static conditions or subjected to shear stress (15 dynes/cm2, 10 min) and immunostained with anti-YAP antibody together with DAPI.
(H) Quantification of nuclear fluorescence intensity of YAP relative to that of cytoplasm as in (G) (n = 3 independent experiments, in each of which >100 cells were measured).
(I) A schematic representation of how YAP translocates into the nucleus in response to shear stress. In static ECs YAP is kept in the cytoplasm, at least in part by binding to AMOT. Upon shear stress, a significant population of AMOT preferentially binds to the increased levels of cortical actin bundles. By direct binding to F-actin or by other unknown mechanism, AMOT releases YAP. Released YAP then enters into the nucleus to promote TEAD-dependent transcription.
(J) Projection view of confocal stack fluorescence images of the trunk region in Tg(fli1:Gal4db-TEAD2ΔN-2A-mC);(UAS:GFP);(fli1:Myr-mC) WT (left) and homozygous amotl2afu45 mutant embryos (right) at 30 hpf. Upper panels, GFP images; lower panels, merged images (GFP, green; Myr-mC, red). Arrows indicate ectopic GFP expression in the developing ISVs. Representative images of three independent experiments are shown.
Data are means ± SD in (B), (F), and (H). ∗∗p < 0.01, ∗∗∗p < 0.001; NS, not significant. Scale bars, 10 μm. See also Figure S6.
Reprinted from Developmental Cell, 40, Nakajima, H., Yamamoto, K., Agarwala, S., Terai, K., Fukui, H., Fukuhara, S., Ando, K., Miyazaki, T., Yokota, Y., Schmelzer, E., Belting, H.G., Affolter, M., Lecaudey, V., Mochizuki, N., Flow-Dependent Endothelial YAP Regulation Contributes to Vessel Maintenance, 523-536.e6, Copyright (2017) with permission from Elsevier. Full text @ Dev. Cell