Fig. S6
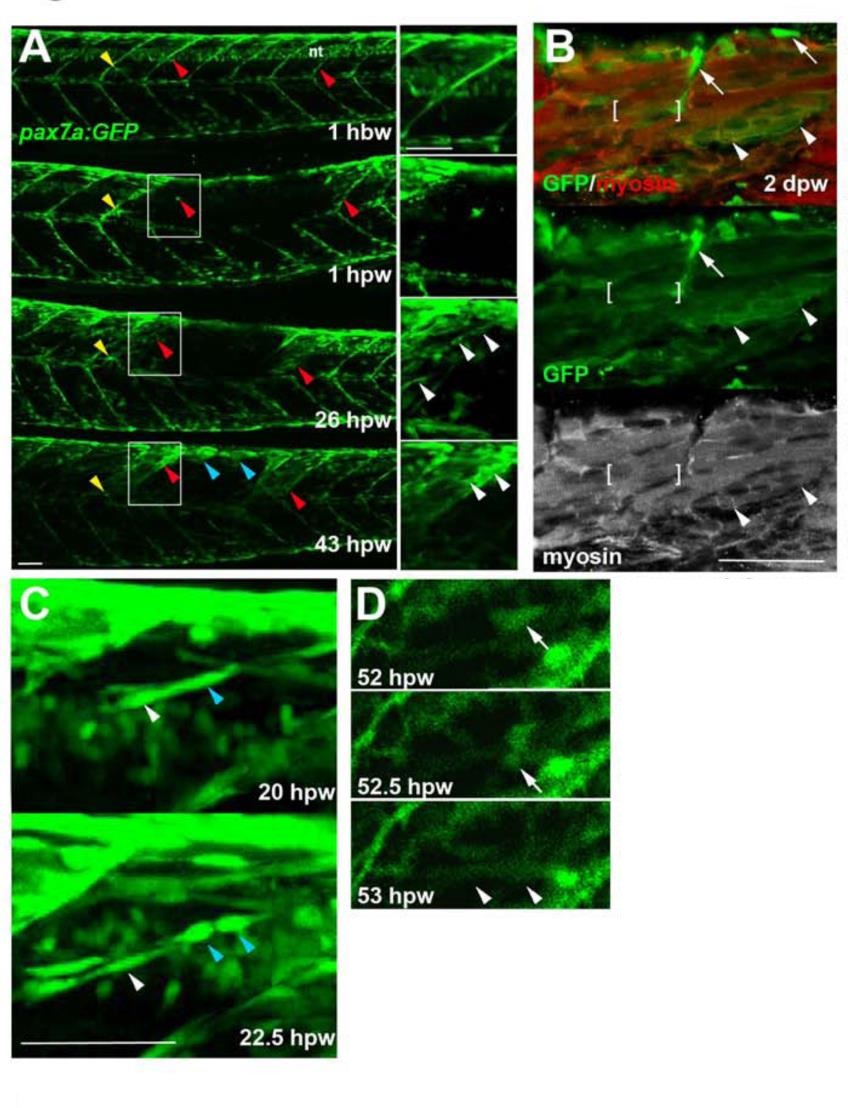
Genetically marked pax7aexpressing cells contribute to regeneration. Pax7a:GFP zebrafish larvae wounded at 3-4 dpf were repeatedly confocally scanned live embedded In agarose (A,e,D) or fixed and stained (8). All larvae are shown in lateral view, anterior to left, dorsal up. Repair of large wounds followed a variable spatiotemporal course, likely reflecting variability in the extent of initial wounding. Whereas in some cases repair seemed faster near to unwounded somites (A), in other cases recovery at the dorsal edge of wounded somites was more rapid and repair progressed in parallel in all somites (see Fig. S7). A. Time lapse of pax7a:GFP;pfe/pfe larva wounded at 4 dpf in the epaxial reg ion of somites 16-20. Prior to wounding (top panel), GFP is present in neural tube (nt), somites borders and a residual xanthophore (yellow arrowheads), which marks the anteriormost wounded somite. Note the recovery of GFP in central myotome of somites that retained GFP+ cells at vertical myosepta (red arrowheads) and spreading from dorsal epaxial edge (blue arrow- heads). Boxed regions, magnified at righl. show elongating GFP+ cells in central somite 16 at 1 dpw (white arrowheads) yielding thin muscle fibers by 2 dpw. In large wounds, most pax7a:GFP signal was lost at the wound site, consistent with ablation of many muscle precursor cells. By 1 dpw, pax7a:GFP+ cells began to re-accumulate from the edge of the wounded region, occupying the centre of wounded somites and seeming to elongate (insets). AI 2 dpw. pax7a:GFP-expressing cells contributed to muscle fibre regeneration, as demonstrated by the appearance of GFP in elongated multinucleate muscle fibres containing myosin heavy chain (compare A with B). B. Immunodeteclion of pax7a:GFP and myosin heavy chain (MyHC) two days after wounding two adjacent somites revealed mononucleate GFPstrongMyHC cells (arrows), thin GFPmediumMyHCweak nascent fibres with unstructured MyHC (arrowheads) and larger GFPweakMyHC+ maturing fibres with recognisable sarcomeric structure (between brackets). The pax7a:GFP signal rapidly weakened as fibres enlarged, often requiring immunodetection to reveal the GFP in maturing regenerated fibres. Strikingly though, the number of pax7a:GFP-labelled cells in somites in the central region of large wounds remained low; most labelled mononucleate cells were present in somites at the wound edge, where new GFP-labelled fibres also first appeared (see A 43 hpw). It therefore appears that pax7a:GFP marked cells at wound edges initially contributed to muscle regeneration. C. Brief time lapse series of short stacks showing pax7a:GFP+ cells dividing (blue arrowhead) and migrating (white arrowhead). Such detailed time-lapse analysis confirmed that pax7a:GFP marked cells migrate and divide, consistent with their re-accumulation in the lesion. D. Brief time-lapse series of single plane showing pax7a:GFP+ cell moving (arrows) and then fusing into a fibre (arrowheads). After entering the wound a proportion of cells fuse into nascent fibers. These results show that Pax7a+ cells at the dorsal and vertical somite border can initiate fibre regeneration. Bars = 50 µm.