Fig. 6
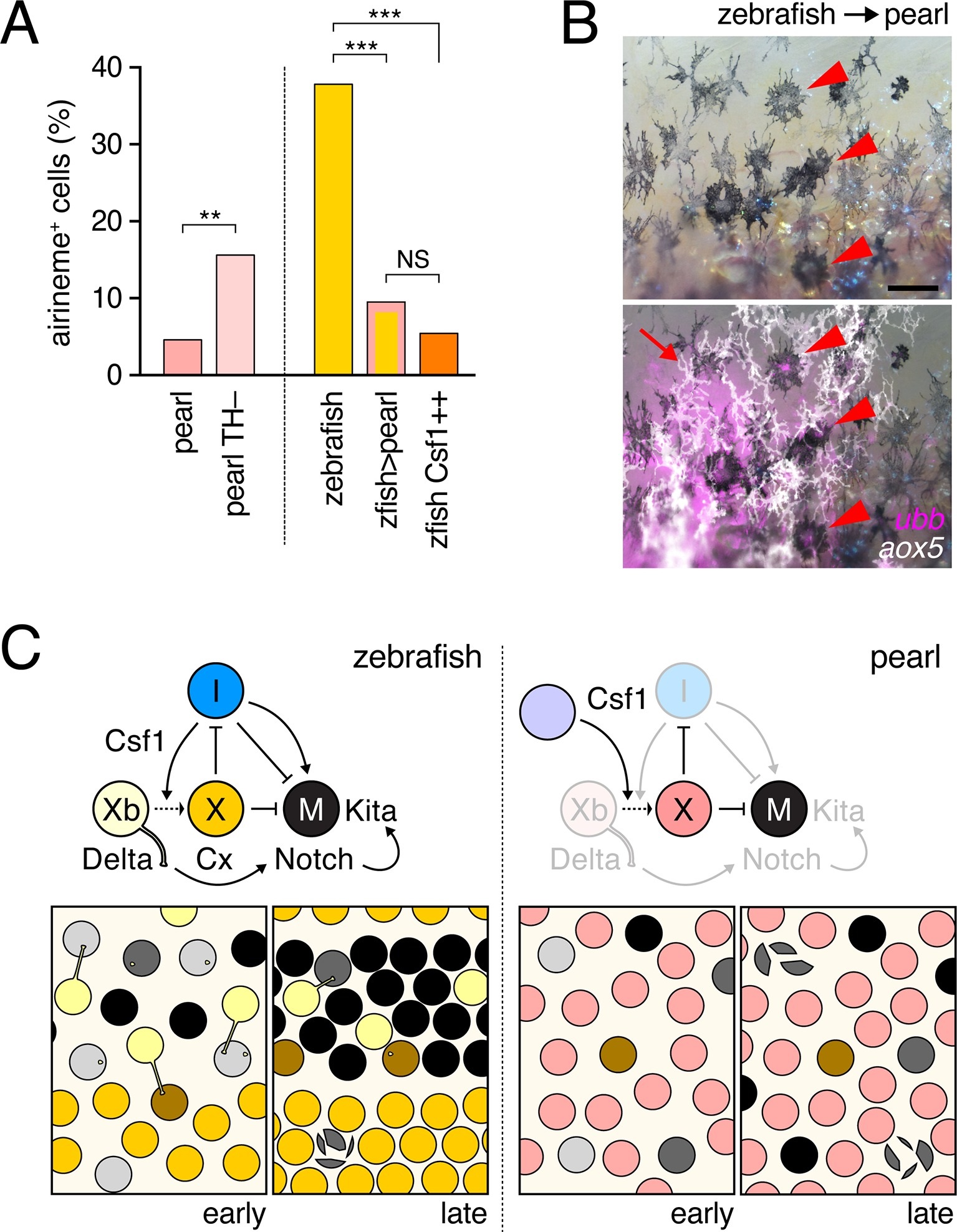
Factors extrinsic to the aox5+ cells inhibit airineme production and signaling in pearl and models for airineme signaling and its evolution.(A) Left, Pearl aox5+ cells extended more airinemes when differentiation-arrested (TH–; χ2=12.5, d.f.=1, p<0.0005, N=412 cells). Right, zebrafish aox5+ cells transplanted to pearl, or receiving excess Csf1 in zebrafish, extended fewer airinemes than comparably staged aox5+ cells in unmanipulated zebrafish (χ2=23.1, 22.1, d.f.=1, 1; p<0.0001, N=846 cells total). (B) In chimeras resulting from transplants of zebrafish donors (aox5:membrane-GFP, ubiquitous ubb:mCherry [Mosimann et al., 2011]) to pearl hosts, zebrafish aox5+ cells (arrow) were typically intermingled with pearl melanophores, as well as ubb+ zebrafish melanophores (arrowheads). (C) Working models for pigment cell interactions and pattern formation in zebrafish (left) and pearl danio (right). In zebrafish, xanthoblasts in stripe regions extend airinemes that signal to melanophores (Xb→M), promoting their clearance from the interstripe during stripe consolidation. Results of this study are consistent with interactions involving xanthoblast airineme dependent Delta (Dlc or possibly Dll4) activation of Notch signaling in melanophores, and the potentiation of Kita-dependent melanophore motility. Nevertheless, these data do not exclude roles for additional modes of Delta–Notch signaling, or the possibilities that airinemes transduce additional signals, or signals that cannot be distinguished from the Delta–Notch pathway using the experimental paradigms here employed. Analyses of mutant zebrafish further support roles for Cx41.8 in contributing to airineme-dependent communication, through modulation of target specificity or xanthophore lineage differentiation. In addition to xanthoblast–melanophore interactions, iridophores have attractive and repulsive effects on melanophores (I→M; I⊣M) (Frohnhofer et al., 2013; Patterson and Parichy, 2013) and express Csf1, promoting the differentiation of xanthophores (Xb→X) at the interstripe (Patterson and Parichy, 2013). Differentiated xanthophores repel melanophores (X⊣M) during normal development (Nakamasu et al., 2009) and are capable of repressing iridophore organization (X⊣I) [for details, see: (Patterson et al., 2014)]. In pearl danio, Csf1 is expressed at elevated levels by cells other than iridophores and this drives earlier and broader xanthophore differentiation than in zebrafish (Patterson et al., 2014). Precocious, widespread differentiation of xanthophores likely limits directional cues available to melanophores while simultaneously curtailing the potential for airineme signaling, as airineme competent xanthoblasts are depleted. Tissue contexts (lower panels) also show eventual death of some melanophores remaining in the interstripe in zebrafish (Parichy et al., 2000; Parichy and Turner, 2003b) and the higher overall incidence of melanophore death in pearl danio (Quigley et al., 2005); iridophores are omitted for clarity. Scale bar: 50 µm (B).