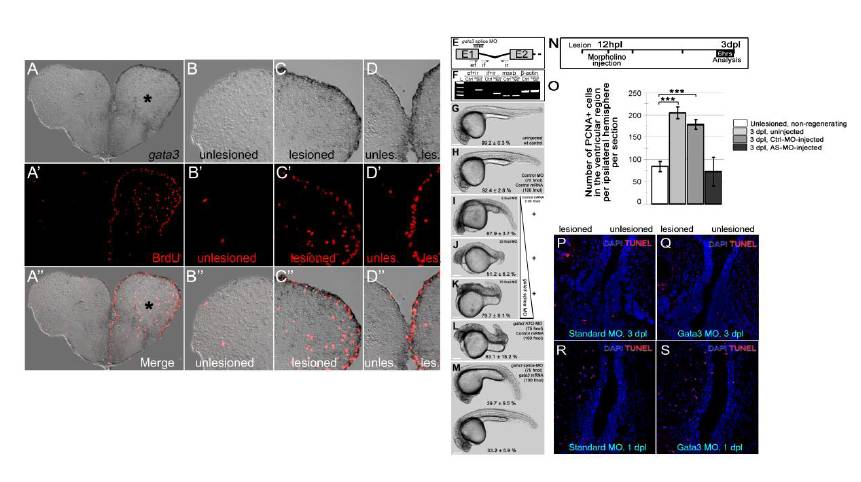
Fig. S2 gata3 expression overlaps with cell proliferation at the ventricular region. (A) gata3 in situ hybridization (ISH) on 3 day-post-lesion (dpl) telencephalon section. Asterisk: lesion site. (A′) BrdU immunohistochemistry (IHC) on the same section in a. BrdU was applied for 4 hours before sacrificing the animal. Note the extensive cell proliferation at the ventricular region of the lesioned hemisphere. (A′ ′) Merge image of ISH and IHC. (B-B′ ′) High-magnification image of the dorsolateral part of the unlesioned hemisphere. No reactive cell proliferation is observed. (C-C′ ′) High-magnification image of the dorsolateral part of the lesioned hemisphere. gata3 and BrdU are overlapping and predominantly at the ventricular region. (D-D′ ′) High-magnification image of the dorsomedial part of the telencephalon. Lesioned hemisphere is to the right. Note the dramatic increase in the cell proliferation after lesion. gata3 overlaps to proliferating ventricular cells. (E) The splice-morpholino oligonucleotide targets the first exon-intron boundary of the gata3 pre-mRNA. ef, if, and ir denotes PCR primers for amplifying the retained intron. (F) Unlike control morpholino-injected embryos, gata3-morphants retain the first gata3 intron. Pre-mRNAs of msxb, a transcription factor, and β-actin, a housekeeping gene, are not affected by gata3 splice morpholino. (G) Uninjected control embryos. (H) Control-morpholino- and control mRNA-injected embryos. (I) 5-fmol of splice-blocking gata3 morpholinos causes defective axial patterning. (J) 25-fmol of splice-blocking gata3 morpholinos causes defective axial elongation. (K) 75-fmol of splice-blocking gata3 morpholinos causes dorsalized embryos with defective axial patterning, elongation and head formation. (L) Translation-blocking ATG-morpholino results in dorsalized embryos with defective axial elongation similar to the same-dosage splice-blocking morpholinos. (M) The morphant phenotypes can largely be rescued by co-injection of full-length gata3 mRNA (39.7±9.5 % of the clutch shows axial elongation but smaller heads and defects in yolk-tube extension; 33.2±5.9 % of the clutch shows normal axial elongation and head formation). Scale bars: 250 μm. n e 100 embryos for each category. All embryos are at 24 hpf. (N) Morpholinos were injected at 12 hpl and telencephalons were analyzed at 3 dpl by PCNA immunostaining. (O) Quantification of the number of PCNA-positive cells in the ventricular region of unlesioned and 3 dpl brains – uninjected, control morpholino-injected and gata3 morpholino-injected. Knocking-down gata3 reduces the number of PCNA-positive cells to control levels. n = 6 fish for every set, bars are ± s.e.m. (P-Q) Knocking down gata3 does not lead to apoptosis. (P) TUNEL staining on 3 dpl telencephalon sections injected with control morpholino. (Q) TUNEL staining on 3 dpl telencephalon sections injected with gata3 antisense morpholino. (R) TUNEL staining on 1 dpl telencephalon sections injected with control morpholino. (S) TUNEL staining on 1 dpl telencephalon sections injected with gata3 antisense morpholino.
Reprinted from Developmental Cell, 23(6), Kizil, C., Kyritsis, N., Dudczig, S., Kroehne, V., Freudenreich, D., Kaslin, J., and Brand, M., Regenerative Neurogenesis from Neural Progenitor Cells Requires Injury-Induced Expression of Gata3, 1230-1237, Copyright (2012) with permission from Elsevier. Full text @ Dev. Cell