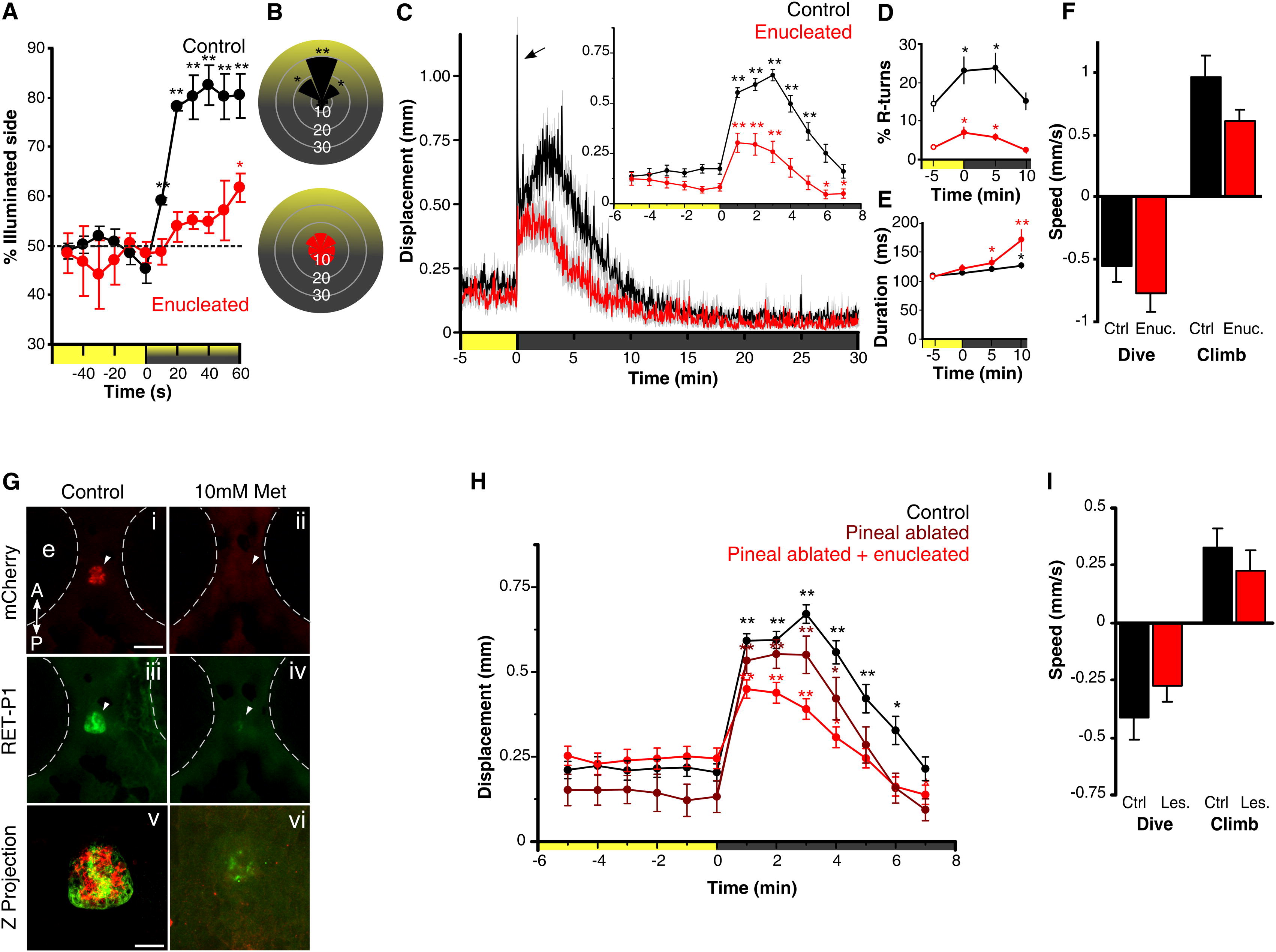
Fig. 1 Light-Driven Behavior in Larval Zebrafish without Eyes or Pineal(A) Attraction of control and enucleated larvae to a phototaxis stimulus, measured by the percent of larvae observed on the illuminated side of the testing arena over time. Enucleated larvae exhibit a gradual shift to the illuminated side of the arena (symbols show one-sample t test to 50%; n = 4 groups of 15 larvae). Larval positions were recorded every second and then averaged over 10 s for each time point. Color along x axis indicates light condition.(B) Larval body orientation during exposure to a phototaxis stimulus. A significant proportion of control larvae exhibit a “head-on” orientation toward the spotlight (one-way ANOVA; F7, 24 = 51.21, p < 0.001; comparisons are Tukey’s post hoc), whereas enucleated larvae show no bias in body orientation (ANOVA; F7, 24 = 1.73, p = 0.15; n = 4 groups of 15 larvae). Data represent mean proportion of larvae oriented relative to the target light over 1 min.(C) Locomotor activity during dark-induced VMR. Arrow indicates O-bend spike observed only in controls. Inset: enucleated larvae significantly increase activity following light extinction (repeated-measures ANOVA; F2.5, 88.7 = 16.57, p < 0.001; n = 36 larvae). Data represent the mean activity for the preceding minute. Color along x axis indicates light condition. Pairwise comparisons are to the baseline time point at 5 min.(D and E) Kinematic analysis of VMR. Enucleated larvae (red) retain elevated R-turn initiation frequency (D; repeated-measures ANOVA; F2.1, 56.6 = 4.63, p = 0.013) and swim bout duration (E; repeated-measures ANOVA; F3, 40 = 6.41, p = 0.001) as seen in controls. Data represent the mean of observations during the first 16 s following each time point. Pairwise comparisons are to the baseline measurement at 5 min (empty circles) (control: n = 18 groups of 10 larvae; enucleated: n = 28 groups of 10 larvae).(F) Diving and climbing speed during VMR. In either response, enucleated larvae were not significantly different from controls (dive: t test, p = 0.27; climb: t test, p = 0.07; n = 6 groups of 5 larvae). Additionally, speed in all conditions is significantly different from 0 (one-sample t test, p < 0.005). Data represent mean vertical swim speed over the first 20 s of dive and ascent.(G) Nitroreductase-mediated ablation of the pineal. Dorsal views at 6 days postfertilization (dpf) are shown. (i–iv) Epifluorescence images of the pineal (arrow) in untreated and metronidazole (Met)-treated Tg(tph2:NfsB-mCherry)y227 larvae with anti-mCherry (red; i and ii) and anti-RET-P1 (green; iii and iv). Scale bar represents 100 μm. (v and vi) Confocal z projections (mCherry + RET-P1) showing concurrent nitroreductase and opsin expression in the pineal in untreated (v) and Met-treated (vi) larvae. Scale bar represents 25 μm.(H) VMR in enucleated, pineal-ablated larvae. Both pineal-ablated and pineal-ablated + enucleated larvae show a robust VMR following light extinction (repeated-measures ANOVA; F3.8,131.3 = 32.44, p < 0.001) (control and pineal-ablated + enucleated: n = 36 larvae; pineal-ablated: n = 26 larvae). Data represent mean activity for the preceding minute. Pairwise comparisons are to baseline time point 5 min.(I) Diving and climbing speed of enucleated, pineal-ablated larvae during VMR. In either response, lesioned larvae were not significantly different from controls (t test; dive: p = 0.26; climb: p = 0.42). Mean speed of dive and ascent for both groups is significantly different from zero (one-sample t test, p < 0.005; n = 14 groups of 5 larvae). Data represent mean swim speed over the first 20 s of dive and ascent.For all panels, error bars show SEM; p < 0.05, p < 0.01. See also Figure S1.
Image
Figure Caption
Figure Data
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Curr. Biol.