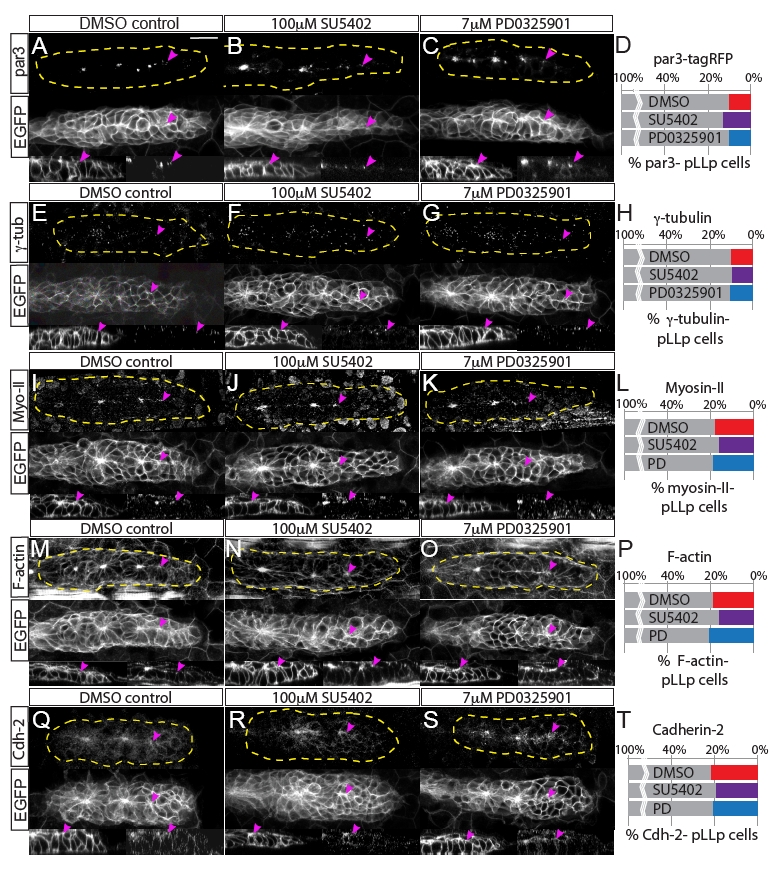
Fig. S4
Fgfr-Ras-MAPK signaling does not disrupt localization of polarity molecules or the actomyosin cytoskeleton. Analyses of polarity and cytoskeletal recruitment in the pLLp. (A-C) Par3 localization was examined by overexpression of par3-TagRFP. (E-G,I-K,M-O,Q-S) Immunohistochemistry was used to examine localization of (E-G) γ-tubulin, (I-K) Myosin II, (M-O) F-actin and (Q-S) Cadherin 2. Each panel shows immunohistochemistry for the indicated molecule (top), claudinB:EGFP (middle) and sagittal views of the leading edge (claudinB:EGFP, bottom left; and immunolabeling against indicated molecule, bottom right). All molecules are properly localized to the apical domains of cells in the leading region of the pLLp, including pLLps in embryos treated with Fgfr or MAPKK inhibitors (2-hour treatment, 28-30 hpf). Pink arrowheads indicate the most caudal accumulation of each molecule. (D,H,L,P,T) Quantification of recruitment of polarity/cytoskeletal molecules to the apical domains of pLLp cells, showing the percentage of pLLp cells that do not display apically localized Par3-TagRFP, γ-tubulin, F-actin, Myosin II or Cadherin 2. Percentages were derived by counting the number of cells caudal to a given distalmost signal (i.e. the number of leading cells that lacked the signal) and normalizing to the total number of cells in the pLLp. There is no significant difference in the recruitment of any of these molecules (one-way ANOVA). Scale bar: 20 μm.