Fig. S3
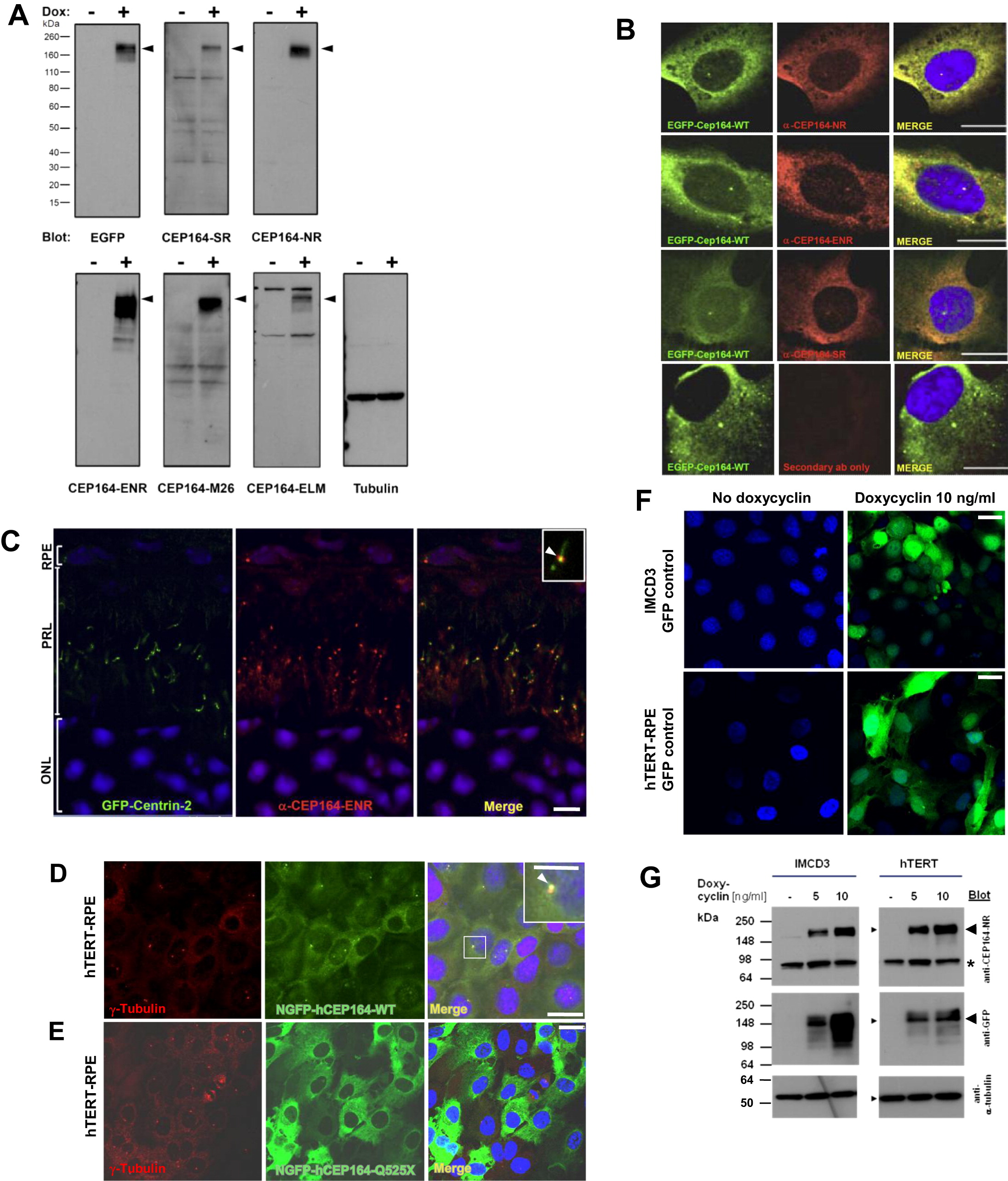
Subcellular Localization of CEP164 (A) Characterization of anti-CEP164 antibodies by immunoblotting. IMCD3 cells that doxycycline-inducibly express GFP-CEP164 isoform 1 were induced with (+) or without (-) 10 ng/ml doxycyclin for 20 hr. 20 μg of RIPA extracted cell lysates were subjected to SDS-PAGE and blotted with the indicated antibodies. Molecular weight is shown in kDa. Note that anti-CEP164 antibodies recognize a band compatible with the size of EGFP-CEP164 isoform 1 (191 kDa; arrow heads). Loading control (on right) uses an anti-αtubulin antibody. (B) Characterization of α-CEP164 antibodies by overexpression of N terminally GFP-labeled human isoform 1 wild-type construct EGFP-CEP164-WT and immunofluorescence in cell lines. Cells were costained with antibodies α-CEP164-NR, -ENR, and -SR (red), respectively, demonstrating colocalization (Merge) with N-EGFP-hCEP164-WT (green). The secondary antibody alone (lower panels) does not result in a signal. Scale bars, 5 μm. (C) CEP164 in GFP-Centrin-2 mouse photoreceptors. In photoreceptors of centrin-2-GFP mice, immunofluorescence using anti-CEP164-ENR antibody detects Cep164 at basal bodies/mother centrioles (visible as a single red dot), whereas centrin-2-GFP is expressed at both centrioles, mother and daughter. Inset shows enlargement of a representative centrosome at 3-fold higher magnification. Nuclei are stained with DAPI. Scale bars, 5 μm. ONL, outer nuclear layer; PRL, photoreceptor layer; RPE, retinal pigment epithelium. (D–G) Expression of mutant CEP164 in hTERT-RPE cells abrogates localization to centrosomes. (D) Doxycyclin (Dox)-inducible overexpression of N terminally GFP-tagged human full-length CEP164 wild-type isoform 1 (NGFP-hCEP164-WT) in hTERT-RPE (human retinal pigment epithelium) cells demonstrates, in addition to a cytoplasmic expression pattern, localization at centrosomes. (E) In contrast, the signal at centrosomes is abrogated upon overexpression of an N terminally GFP-tagged truncated CEP164 construct (NGFP-CEP164-Q525X) representing the mutation p.Q525X occurring in NPHP-RC family F59. Similar data was obtained upon CEP164 expression in IMCD3 cells (see also Figures 3B–3D). (F) Expression of GFP alone as a negative control yielded no centrosomal expression in IMCD3 or hTERT-RPE cells. hTERT-RPE cells were stably transfected with the CEP164 constructs in a retroviral vector for doxycyclin-inducible expression (pRetroX-Tight-Pur). Following selection with puromycin for 2 weeks, cells were induced with 10 ng/ml of doxycyclin for 20 hr. Cells were then costained with γ-tubulin (red) for the colocalization with NGFP-hCEP164-WT fusion proteins (green) (see also Figures 3B–3D). Scale bars, 10 μm. (G) Immunoblot of IMCD3 and hTERT-RPE cells expressing inducible GFP-CEP164 constructs confirmed doxycyclin-inducible NGFP-CEP164 fusion protein expression (<191 kDa) both with α-CEP164-NR and α-GFP antibody blotting (arrow heads). The band at <85 kDa (asterisk) may represent the endogenous isoform 2 of CEP164 (84 kDa). IMCD3 and hTERT-RPE cells were stably transfected with the N terminally tagged-GFP-CEP164 construct in a retroviral vector (pRetroX-Tight-Pur) for doxycyclin-inducible expression. Cells were selected on puromycin for 2 weeks. Both cell lines were plated and treated with the indicated doses of doxycycline and lyzed with RIPA buffer 24 hr after induction. A total of 50 μg of cell lysates were loaded onto SDS-PAGE and blotted with the antibodies indicated. The same membranes were reprobed with anti-α-tubulin antibody as an indicator of equal loading (GFP-blotted membrane shown) (see also Figure 3). See also Figure 3.
Reprinted from Cell, 150(3), Chaki, M., Airik, R., Ghosh, A.K., Giles, R.H., Chen, R., Slaats, G.G., Wang, H., Hurd, T.W., Zhou, W., Cluckey, A., Gee, H.Y., Ramaswami, G., Hong, C.J., Hamilton, B.A., Cervenka, I., Ganji, R.S., Bryja, V., Arts, H.H., van Reeuwijk, J., Oud, M.M., Letteboer, S.J., Roepman, R., Husson, H., Ibraghimov-Beskrovnaya, O., Yasunaga, T., Walz, G., Eley, L., Sayer, J.A., Schermer, B., Liebau, M.C., Benzing, T., Le Corre, S., Drummond, I., Janssen, S., Allen, S.J., Natarajan, S., O'Toole, J.F., Attanasio, M., Saunier, S., Antignac, C., Koenekoop, R.K., Ren, H., Lopez, I., Nayir, A., Stoetzel, C., Dollfus, H., Massoudi, R., Gleeson, J.G., Andreoli, S.P., Doherty, D.G., Lindstrad, A., Golzio, C., Katsanis, N., Pape, L., Abboud, E.B., Al-Rajhi, A.A., Lewis, R.A., Omran, H., Lee, E.Y., Wang, S., Sekiguchi, J.M., Saunders, R., Johnson, C.A., Garner, E., Vanselow, K., Andersen, J.S., Shlomai, J., Nurnberg, G., Nurnberg, P., Levy, S., Smogorzewska, A., Otto, E.A., and Hildebrandt, F., Exome Capture Reveals ZNF423 and CEP164 Mutations, Linking Renal Ciliopathies to DNA Damage Response Signaling, 533-548, Copyright (2012) with permission from Elsevier. Full text @ Cell