Fig. S6
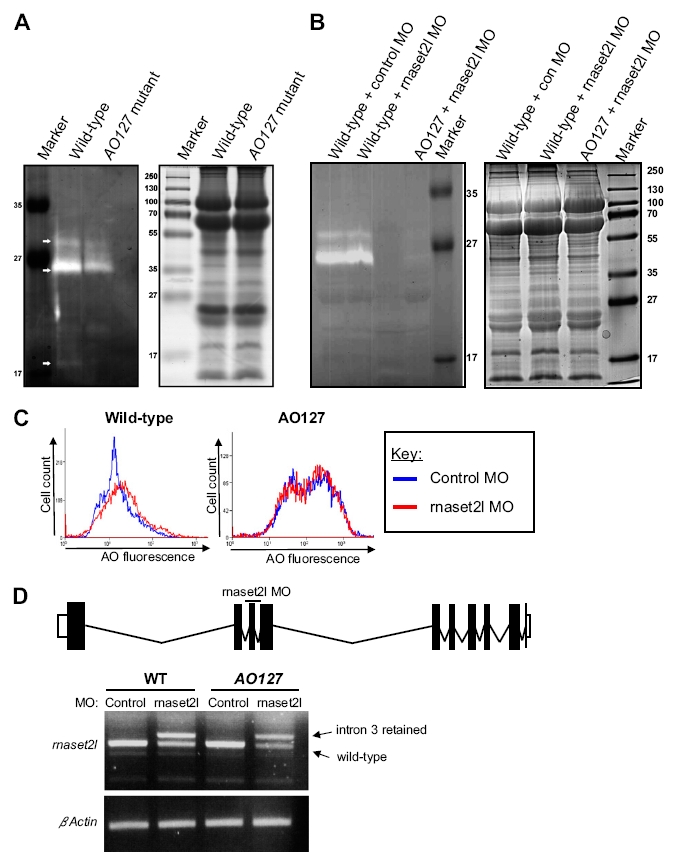
Decreased RNase T2 activity in AO127 mutants and redundancy with rnaset2l. (A) Left: In-gel activity assay was performed at pH 6.0 to analyze RNase T2 activity in protein extracts from WT and AO127 mutant embryos at 3 d after fertilization. White arrows indicate species present in only WT or greatly diminished in AO127 mutants. Note the different molecular weight species result from glycosylation (1) and/or possibly from proteolytic processing of RNase T2 (2). Right: SDS/PAGE gels run in parallel and stained with Coomassie blue to verify loading amounts and protein quality. (B) As in A, only samples prepared from embryos injected with a standard control oligonucleotide (control MO) or antisense oligonucleotide targeting rnaset2l (rnaset2l MO) are shown. (C) Embryos at 3 d after fertilization injected with control MO or rnaset2l MO were stained with AO, disaggregated, and subjected to flow cytometry. (D) Depiction of exon–intron structure of the rnaset2l gene and the position of a MO designed to interrupt splicing of exon 3. RT-PCR revealed the presence of mRNA retaining intron 3 from animals injected with the rnaset2l MO. Residual WT mRNA is presumably maternal. β-Actin serves as a control for RNA integrity and equal input cDNA.
1. Hillwig MS, et al. (2009) Zebrafish RNase T2 genes and the evolution of secretory ribonucleases in animals. BMC Evol Biol 9:170.
2. Campomenosi P, et al. (2006) Characterization of RNASET2, the first human member of the Rh/T2/S family of glycoproteins. Arch Biochem Biophys 449:17–26.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Proc. Natl. Acad. Sci. USA