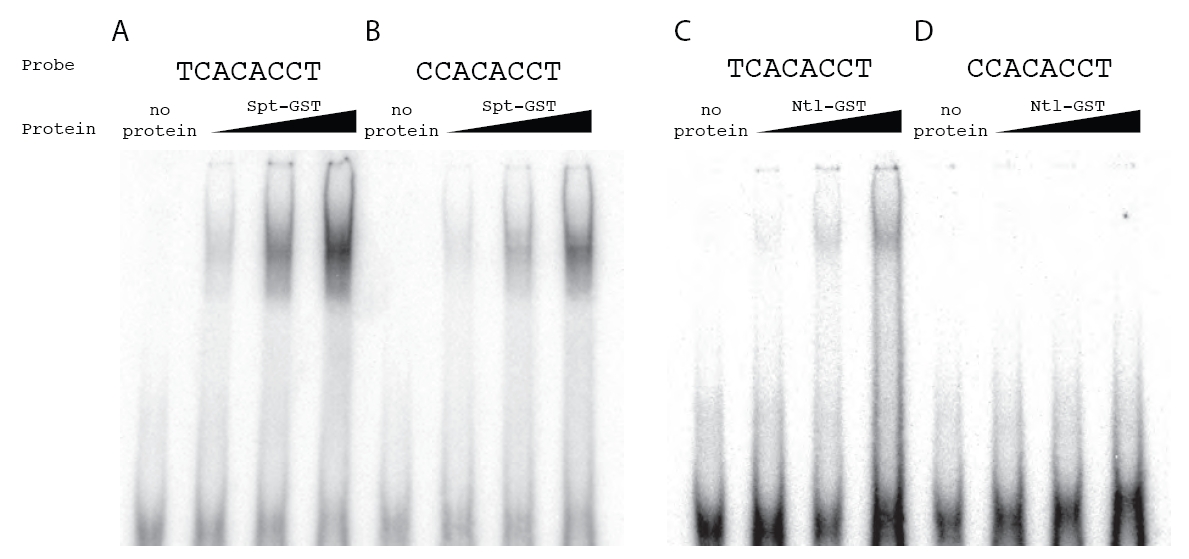
Fig. S3 Spt-GST and Ntl-GST have differential in vitro binding activities. To extend the observation from the SELEX assays that Spt bound a C at position 1 in 25% of the sequenced oligos where as Ntl bound a C at this position in only 2% of the sequenced oligos (Fig. 2), we directly compared the ability of Spt and Ntl to bind TCACACCT versus CCACACCT. Shown are gel mobility shift assays using radiolabeled probes and increasing amounts of bacterially produced Spt-GST and Ntl-GST fusion proteins. Complexes were fractionated on native polyacrylamide gels. Spt-GST bound both probes, but bound TCACACCT probe (A) with higher affinity than the CCACACCT probe (B). As predicted, Ntl-GST also bound the TCACACCT probe (C), but we were unable to detect any Ntl-GST binding to the CCACACCT probe (D). This motif difference has been previously noted between Brachury and Tbx6 in mouse (White and Chapman, 2005), and our data are consistent with previous SELEX experiments with T-box factors, which show that Ntl orthologs do not select sequences with a C at position 1 (Kispert and Herrmann, 1993; Conlon et al., 2001) where as all other tested T-box factors are able to bind oligomers with a C at this position (Conlon et al., 2001; Ghosh et al., 2001; Yagi, 2005; White and Chapman, 2005).
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Development