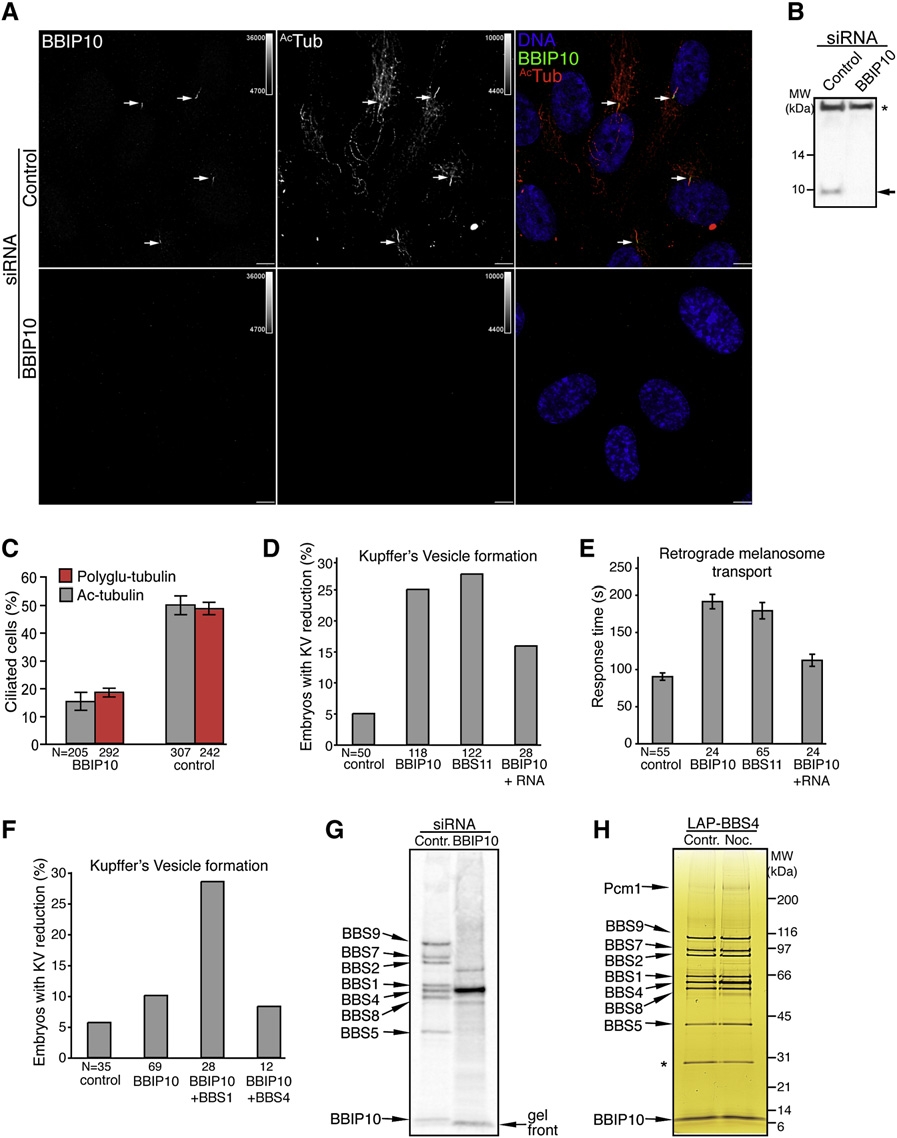
Fig. 2 BBIP10 Is Essential for Ciliogenesis and BBSome Assembly
(A) BBIP10 is required for primary cilium formation in RPE cells. RPE cells were transfected with BBIP10 or control siRNA for 72 hr, serum starved for the last 48 hr, and fixed. Indirect immunofluorescence was performed with anti-BBIP10 and anti-ac-tubulin antibodies. Arrows indicate primary cilia. Scale bars are 10 μm.
(B) Validation of the anti-BBIP10 antibody by western blot. Whole-cell lysates of BBIP10 or control siRNA-transfected RPE cells were immunobloted for BBIP10 (band marked with an arrow). The nonspecific band marked with an astersik was used as a loading control. The complete blot is presented in Figure S4.
(C) Depletion of BBIP10 prevents ciliogenesis. RPE cells were prepared as in described in (A), stained for pericentrin and acetylated or polyglutamylated (GT335) tubulin. Cilia were counted in two independent experiments. Error bars represent SEM.
(D) BBIP10 depletion in zebrafish results in abnormal KV formation. Twenty five percent of the embryos had defects in KV morphology when injected with BBIP10 MO, whereas only 5% of control-injected embryos had such abnormalities. The phenotype could be rescued by coinjection of BBIP10 mRNA (100 ng/μl). BBS11 MO here and in (E) was used as a positive control for zebrafish BBS knockdown defects.
(E) BBIP10 morphants display delayed melanosome retraction. BBIP10 MO-injected embryos retracted melanosomes in 191 s, which is significantly (p < 0.001) delayed compared to 90 s in control-injected embryos. BBIP10 mRNA coinjection decreased transport time to 112 s in BBIP10 morphants. Error bars represent SD.
(F) BBIP10 genetically interacts with BBS1. Injection of a low dose of BBIP10 generates KV defects in 10% of embryos, whereas coinjection of a low dose of BBS1 MO with a low dose of BBIP10 MO increased the number of embryos with KV abnormalities to 28.6%. A low dose of BBIP10 coinjected with BBS4 MO did not produce such a change.
(G) The BBSome fails to form in the absence of BBIP10. The BBSome was purified by tandem affinity purification from [35S]-methionine RPE-[LAPBBS4] cells transfected with control or BBIP10 siRNA. Final eluates were resolved on a 4%–12% NuPAGE gel and blotted on PVDF membrane, which was exposed to a phosphostorage screen. The identity of the bands on the scan was inferred from comparison with a silver-stained gel (Figure 1A). The gel front is marked to help distinguish it from the BBIP10 band.
(H) Microtubules are not required for BBSome maintanence. RPE-[LAPBBS4] cells were serum starved for 48 hr and treated with 0.16 μM nocodazole or DMSO control for 18 hr. The BBSome was isolated, resolved on a 4%–12% NuPAGE gel, and silver stained. The asterisk marks the contaminating TEV protease band.
Reprinted from Developmental Cell, 15(6), Loktev, A.V., Zhang, Q., Beck, J.S., Searby, C.C., Scheetz, T.E., Bazan, J.F., Slusarski, D.C., Sheffield, V.C., Jackson, P.K., and Nachury, M.V., A BBSome Subunit Links Ciliogenesis, Microtubule Stability, and Acetylation, 854-865, Copyright (2008) with permission from Elsevier. Full text @ Dev. Cell