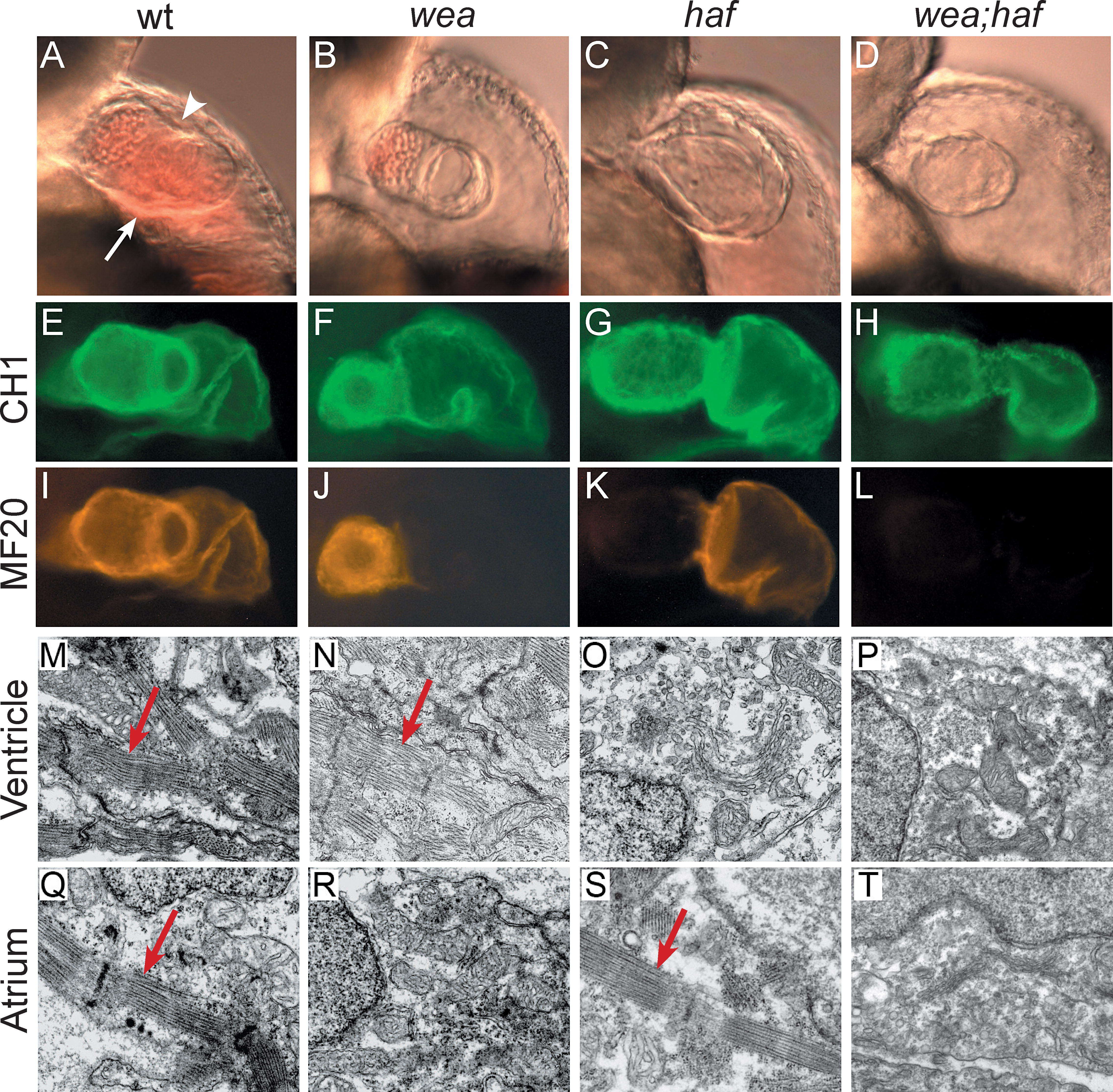
Fig. 3 Mutation of wea and haf Cause Chamber-Specific Contractility Defects. Analysis of wea, haf, and wea;haf double mutants at 48 hpf reveals abnormal ventricular morphology and chamber-specific sarcomere deficiencies. hafsk24 and weam58 are recessive mutations that independently segregate; intercrosses of fish doubly heterozygous for wea and haf produce a 9:3:3:1 ratio of wild type:wea:haf:wea;haf. (A–D) Lateral views of live embryos, anterior to the top, ventricular plane of focus. (A) The wild-type (wt) ventricle possesses distinct concave (arrowhead) and convex (arrow) curvatures. (B) In contrast, the wea ventricle appears small, with less-pronounced curvatures. (C) The haf ventricle appears large and distended, with a notable separation between the myocardium and endocardium. (D) The wea;haf ventricle is small, with relatively spherical contours. (E–L) Whole-mount immunofluorescence detecting tropomyosin (CH1; green) and sarcomeric myosin heavy chain (MF20; red). Lateral views, ventricle to the left. Tropomyosin is present in all cardiomyocytes (E–H). In contrast, myosin heavy chain appears absent from the wea atrium (J), haf ventricle (K), and both chambers of the wea;haf heart (L). (M–T) Ultrastructural analysis of cardiomyocytes by transmission electron microscopy. Normal myofibril arrays (arrows) are present in wt chambers (M and Q), in the wea ventricle (N), and in the haf atrium (S). Organized myofibril arrays are absent from the wea atrium (R), the haf ventricle (O), and both chambers of the wea;haf heart (P and T).
Image
Figure Caption
Figure Data
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ PLoS Biol.