- Title
-
The nieuwkoid/dharma homeobox gene is essential for bmp2b repression in the zebrafish pregastrula
- Authors
- Koos, D.S. and Ho, R.K.
- Source
- Full text @ Dev. Biol.
|
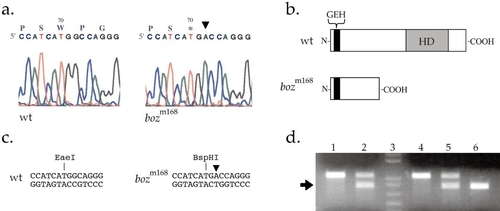
The bozozok locus encodes the Nieuwkoid/Dharma homeodomain protein. (a) Comparison of the genomic sequence of nieuwkoid/dharma from DNA isolated from homozygous wild type (*AB strain) and homozygous bozm168 indicates a single base-pair substitution in the nieuwkoid/dharma ORF that changes W70 to an opal stop codon. Asterisk denotes the stop codon. Arrowhead denotes the mutant base substitution. (b) Schematic depicting the conceptually translated 192-amino-acid product derived from the AB allele and the severely truncated 69-aa product derived from the bozm168 allele. The gray-shaded box denotes the homeodomain and the black-shaded box denotes the GEH motif in the amino-terminal. (c) The molecular lesion in the bozm168 allele generates a restriction fragment length polymorphism in which the single base-pair substitution TGG → TGA destroys an EaeI recognition site and generates a BspHI site. (d) nieuwkoid/dharma is linked to bozm168, as shown by segregation of a BspHI restriction polymorphism in nieuwkoid/dharma sequences amplified from genomic DNAs from mapping crosses. A 1.1-kb PCR-amplified product from the nieuwkoid/dharma sequence is not cleaved by BspHI digestion in wild type. The bozm168 mutant version is cleaved by BspHI digestion, reducing the fragment to 0.8 kb (arrow). Lane 1, homozygous wild-type G0 fish (*AB strain); lane 2, heterozygous |
|
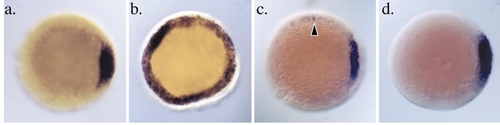
The bozozokm168 allele lacks the ability to induce a functional organizer. All embryos are animal pole facing the viewer at the onset of gastrulation (6 hpf). Dorsal is to the right. Embryos were injected in both blastomeres at the two-cell stage with the indicated synthetic, capped, sense RNA (20 pg per blastomere). (a) gsc expression in uninjected wild-type embryo (*AB). gsc expression is confined to a small region of the dorsal margin in the cells of the forming shield. (b) Radial expression of gsc in the entire margin of embryos following injection of wild-type nieuwkoid/dharma RNA into both blastomeres at the two-cell stage. (c) BOZ.M1 RNA, an engineered allele of nieuwkoid/ dharma containing the bozm168 lesion (W70 to opal), can only weakly induce gsc expression when injected into both blastomeres at the two-cell stage. Arrowhead highlights ectopic weak expression of gsc. (d) Injection of BOZ.M3 RNA, a recombinant allele of nieuwkoid/ dharma that contains coding sequence encoding only the N-terminal 85 amino acids of Nieuwkoid/Dharma, fails to induce ectopic gsc expression when injected as in (b) and (c). |
|
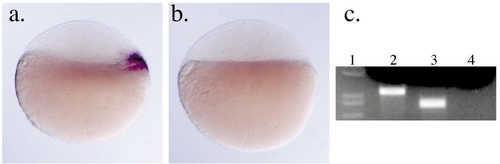
Autoregulation of bozozok prior to the onset of gastrulation. nieuwkoid/dharma RNA is present in wild-type embryos (a) but not in homozygous bozm168 embryos (b) as detected by in situ hybridization at sphere stage (4 hpf). (c) Post-in situ genotyping of embryos in (a) and (b) using PCR and digestion with BspHI. Lane 1, size marker; lane 2, homozygous wild-type embryo (*AB strain) shown in (a); lane 3, homozygous bozm168 embryo shown in (b); lane 4, no-template control. |
|
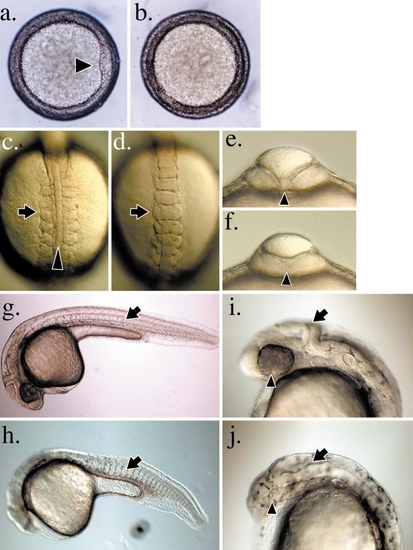
Morphological characterization of the ventralized boz mutant phenotype. (a) Animal pole view of wild-type embryo at the onset of gastrulation (6 hpf). A condensation of cells (arrowhead) denotes the forming shield on the dorsal side of the embryo. (b) Severely affected homozygous bozm168 mutant embryos fail to form a shield. (c and d) Dorsal views of wild-type and homozygous bozm168 mutant embryos, respectively, at 12 hpf. Anterior is toward the top. In wild-type embryos the notochord (arrowhead) is present in the midline separating the lateral blocks of somites (arrow) (c). In homozygous bozm168 mutant embryos (d), the notochord is absent, and the somites are fused across the dorsal midline. (e and f) Optical section of the embryos in (c) and (d), respectively, at the level of the first somite. View is from the anterior. The notochord (arrowhead) is present in wild-type embryos (e) and absent in homozygous bozm168 mutant embryos (f). (g and h) Profiles of wild-type and homozygous bozm168 mutant embryos, respectively, at 26 hpf. Anterior is to the left. Dorsal midline structures such as notochord and floorplate (arrow) are present in wild-type embryos (g), but lacking in homozygous bozm168 mutant embryos (h). (i and j) Profile view of the head region of wild-type and homozygous bozm168 mutant embryos, respectively, at 26 hpf. (j) Homozygous bozm168 mutant embryos often lack differentiated eyes (arrowhead) and display a loss of rostral forebrain neural fates and aberrant patterning of the midbrain region (arrow). |
|
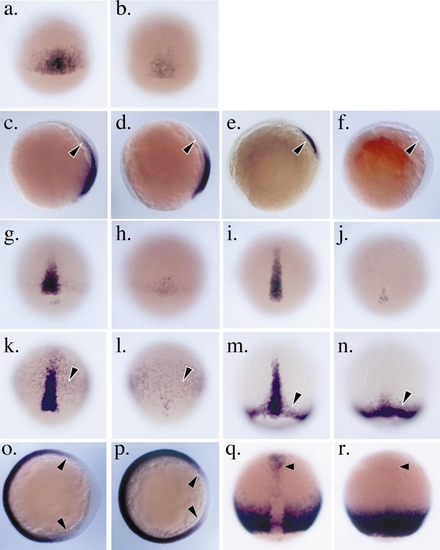
Impaired dorsal specification boz mutant gastrulae. Analysis marker gene expression in wild-type (a, c, e, g, i, k, m, o, q) and homozygous bozm168 mutant (b, d, f, h, j, l, n, p, r) embryos during gastrulation. Embryos in (a) and (b) are staged at the onset of gastrulation (6 hpf); embryos in (c–n) are midgastrula stage at 80% epiboly (8 hpf). All embryos are oriented with animal pole toward the top. (a), (b) and (g–n) are dorsal views, (c–f) profile with dorsal to the right. (a) chd expression at the onset of gastrulation (6 hpf) on the dorsal side of wild-type embryo. (b) In homozygous bozm168 mutant embryos chd expression is reduced at the onset of gastrulation. (c) In wild-type embryos at 80% epiboly, chd expression is detected in the mesendoderm in the nascent axis (arrowhead) as well as the lateral ectoderm (d). In homozygous bozm168 mutant embryos, chd expression is reduced or absent in the nascent axis. (e) gsc expression in the wild-type midgastrula is restricted to the cells of the anteriorly migrating prechordal plate (arrowhead). (f) In homozygous bozm168 mutant embryos, gsc expression is reduced or absent. (g, h) In wild-type embryos the expression of flh (g) and twist (i) is detected in dorsal mesendoderm cells that will later give rise to dorsal embryonic structures such as the notochord. In homozygous bozm168 mutant embryos, the dorsal midline expression of flh (h) and twist (j) is dramatically reduced. (k) axial is expressed during gastrulation in the dorsal mesendoderm as well as in the paraxial endoderm (arrowhead) in wild-type embryos. (l) In homozygous bozm168 mutant embryos, the dorsal mesendoderm expression of axial is very reduced; however, the paraxial endoderm expression does not appear affected (arrowhead). (m) In wild-type embryos, ntl is expressed both in the dorsal axial mesoderm and in the involuting ventral lateral mesoderm. (n) In homozygous bozm168 mutant embryos, the dorsal midline expression of ntl is reduced but the ventral lateral expression is unaffected (arrowhead). (o–r) Expanded ventral gene expression in boz mutant gastrulae. (o and p) The domain of eve1 expression is expanded into the dorsal region of the blastoderm in homozygous bozm168 mutant embryos. Embryos are oriented animal pole toward the viewer, with dorsal to the right. (o) In wild-type embryos at 7 hpf, eve1 expression is excluded from the dorsal region and detected in cells along the ventral two-thirds of the blastoderm circumference. (p) In homozygous bozm168 mutant embryos at 7 hpf, the eve1 expression domain extends into the dorsal region of the embryo. (q and r) The expression of tbx16/spt is altered in homozygous bozm168 mutant embryos at 80% epiboly (8 hpf). Dorsal view with the embryos oriented animal pole to the top. (q) In wild-type embryos, tbx16/spt is expressed in the ventral lateral region of the blastoderm margin and excluded from the trunk dorsal midline. tbx16/spt is also expressed in the dorsal anterior prechordal plate mesendoderm (arrowhead). (r) In homozygous bozm168 mutant embryos, tbx16/spt is detected in the trunk dorsal midline and the dorsal anterior expression domain is greatly reduced or absent. |
|
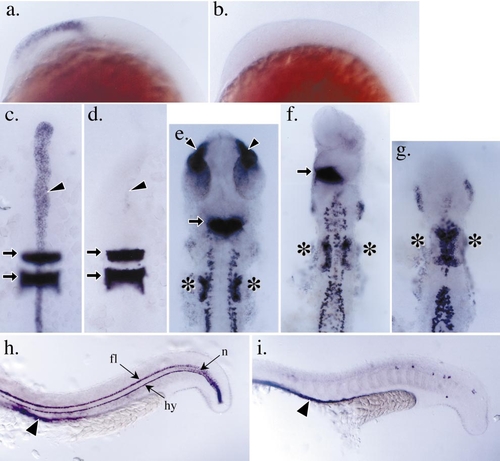
Impaired anterior neural ectoderm patterning in boz mutants. (a and b) Profile views of the developing head region in postgastrula embryos (14 hpf); the expression of emx1 is detected in the developing forebrain region in wild-type embryos (a) and absent in severely affected boz mutant embryos (b). (c and d) The expression of the floorplate and notochord marker, shh, and hindbrain marker, krox20, in the anterior of wild-type and boz mutant embryos, respectively, at 12 hpf. The embryos have been removed from their yolk cell and mounted flat between coverslips. Anterior is toward the top. At this stage in wild-type embryos (c), shh expression (arrowhead) is detected in the dorsal midline and krox20 is expressed in the hindbrain region in presumptive rhombomeres 3 and 5 (arrows). In severely affected boz mutant embryos (d), the expression of shh is reduced or absent, but the development of the hindbrain indicated by the expression of krox20 appears unaffected. (e– g) Expression of paxb in wild-type and boz mutant embryos at 22 hpf. Embryos have been removed from their yolk cell and mounted flat with anterior toward the top. Embryos are aligned with the anterior extent of the pronepherous at the bottom. (e) In the anterior of wild-type embryos, paxb expression is detected in the developing eye stalks (arrowheads), the midbrain–hindbrain boundary (arrow), the otic placodes (asterisks), the neurons, and the pronepherous. (f, g) boz mutant embryos can be grouped into a series of truncations of anterior neuronal fates. For example, mildly affected boz mutants (f) exhibit a loss of eye stalk expression and a reduced midbrain–hindbrain boundary expression of paxb. In severely affected boz embryos (g), these anterior neural deficiencies are enhanced to include a loss of midbrain–hindbrain expression of paxb. Note that the otic placodes in these severely affected mutants appear to be enlarged (asterisks). (h and i) Expression of α-coll2a in the trunk and tail of wild-type and boz mutant embryos, respectively, at 22 hpf. At this stage in wild-type embryos (h), α-coll2a is detected in the floorplate (fl), notochord (n), hypochord (hy), and axial endoderm (arrowhead). In boz mutant embryos (i), the floorplate, notochord, and hypochord are reduced or absent. However, the expression of α-coll2a in the axial endoderm is still detected ventral to the dorsal midline (arrowhead). |
|
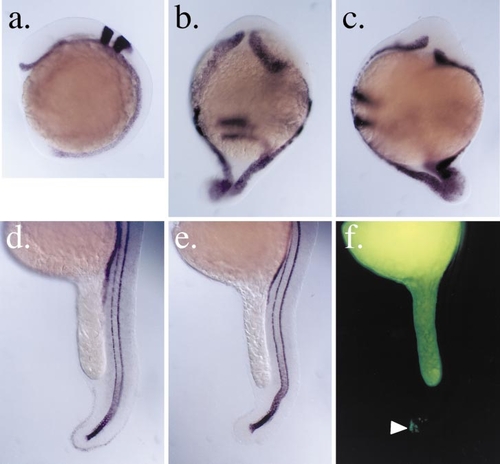
Rescue of the boz ventralized phenotype by expression of bozozok RNA. (a– c) Injection of wild-type nieuwkoid/dharma RNA in early blastomeres of either wild-type or boz mutant embryos led to a hyperdorsalized phenotype. Embryos are at 12-hpf stage. Embryos are oriented anterior to the top. (a) Uninjected wild-type postgastrula embryo stained simultaneously for the notochord and floorplate marker shh and the hindbrain marker krox20. At this stage, shh expression is detected along the entire anteroposterior axis in the cells of the floorplate and notochord. krox20 expression is detected only in the hindbrain region and is restricted to rhombomeres 3 and 5. (b) Injection of wild-type nieuwkoid/dharma RNA in distant blastomeres of eight-cell-stage stage wild-type or boz heterozygote embryos led to the formation of expanded or ectopic dorsal fates as indicated by the ectopic expression of shh and radially expanded krox20 expression. (c) Injection of nieuwkoid/dharma RNA in boz homozygotes as in (b) led to the formation of ectopic dorsal fates. (d–f) Analysis of wild-type and boz mutant embryos following injection of nieuwkoid/dharma RNA directly into the YSL. Wild-type (d) and bozozok mutant (e and f) embryos at 22-hpf stage following injection of nieuwkoid/dharma RNA into the YSL and staining for the expression of α-coll2a. Embryos are in profile and oriented anterior to the top. YSL injection of nieuwkoid/dharma RNA can rescue the formation of dorsal midline tissues in boz homozygotes (e) but does not lead to the formation of hyperdorsalized postgastrula phenotype in either boz mutants (e) or their wild-type (d) and heterozygote siblings. (f) Fluorescence image of rescued boz mutant embryo in (e) revealing the distribution of lineage tracer that was coinjected with the nieuwkoid/dharma RNA. No labeled cells were detected in the midline tissues. However, frequently a small group of labeled cells was detected in the posterior region of the tail (white arrowhead). |
|
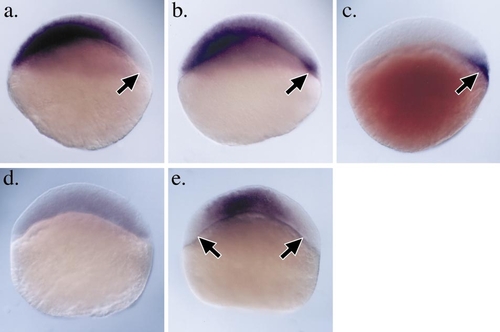
zbmp2b is ectopically expressed in pregastrula boz mutants. Analysis of zbmp2b expression in wild-type and homozygous bozm168 mutant embryos prior to the onset of gastrulation. All embryos are oriented with animal pole toward the top and dorsal to the right. (a) In wild-type embryos prior to the onset of gastrulation at 40% epiboly stage, zbmp2b transcripts are not detectable on the dorsal side of the embryo. The region free of zbmp2b message is complementary to the dorsal blastoderm region that expresses nieuwkoid/dharma, shown for reference in a slightly younger wild-type embryo (c). (b) In contrast, zbmp2b transcripts do not clear completely from the dorsal margin in homozygous bozm168 mutant embryos and the persistent zbmp2b expression is also detected in the dorsal YSL. (d and e) Misexpression of nieuwkoid/dharma RNA is sufficient for the down regulation of zbmp2b expression in wild-type pregastrula embryos. (d) Embryo injected with nieuwkoid/dharma RNA in both blastomeres at the two-cell stage and assayed for zbmp2b expression at 40% epiboly stage. zbmp2b expression is strongly reduced throughout the entire blastoderm. (e) zbmp2b expression at 40% epiboly in wild-type embryo injected with nieuwkoid/dharma RNA in two distant blastomeres at the eight-cell stage. Ectopic and expanded regions of zbmp2b repression are observed (arrows). |
Reprinted from Developmental Biology, 215(2), Koos, D.S. and Ho, R.K., The nieuwkoid/dharma homeobox gene is essential for bmp2b repression in the zebrafish pregastrula, 190-207, Copyright (1999) with permission from Elsevier. Full text @ Dev. Biol.