- Title
-
Zebrafish sparse corresponds to an orthologue of c-kit and is required for the morphogenesis of a subpopulation of melanocytes, but is not essential for hematopoiesis or primordial germ cell development
- Authors
- Parichy, D.M., Rawls, J.F., Pratt, S.J., Whitfield, T.T., and Johnson, S.L.
- Source
- Full text @ Development
|
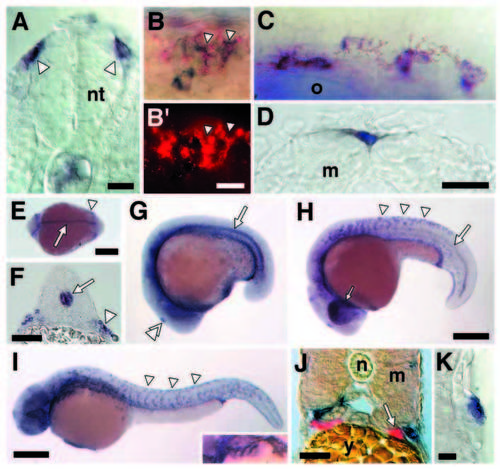
kit is expressed by NC-derived melanoblasts and melanocytes during wild-type zebrafish development. (A) Transverse cryosection showing NC cells (arrowheads) expressing kit as they disperse from the neural tube (nt) (18 hours cryosection). (B) Two-color in situ hybridization reveals cells expressing both kit (blue, in B) and nacre (red, in B, B′) in the dorsolateral NC migratory pathway (24 hours whole mount). (C) Lightly pigmented melanocytes expressing kit dorsal to the otocyst (o) (30 hours whole mount). (D) Fully differentiated melanocyte in the dorsal stripe expressing kit (12 day larva, cryosection); m, myotome (treated with PTU to partially inhibit melanin synthesis and better visualize kit staining). (E-K) kit also is expressed by an array of other cell types. (E) kit expression in notochord (arrow) and lateral mesodermal cells that are hematopoietic precursors and will form the intermediate cell mass (arrowhead; Detrich et al., 1995) (14 hours, whole mount dorsal view, anterior to left). (F) Notochord (arrow) and lateral mesodermal staining (arrowhead; 18 hours, transverse cryosection). (G) Notochord staining and staining in the pineal gland (double arrowhead; 17 hours, whole mount). (H) Dispersing, kit-expressing NC cells in the trunk (arrowheads), retinal staining in the eye (small arrow) and posterior notochord staining (large arrow; 22 hours, whole mount). (I) kitexpressing NC cells in the trunk (arrowheads) and branchial arches (inset; whole mount, 30 hours). (J) Zebrafish PGCs were not observed to express kit between 14 hours and 6.5 days. Shown are merged fluorescence and brightfield views of a transverse cryosection with vasastained PGCs (red) that do not stain for kit (blue, in adjacent melanocytes; 42 hours). n, notochord; y, yolk. (K) kit also is expressed in the lateral line sensory system (transverse cryosection, 3 day larva). In contrast to a Xenopus kit-like receptor (Baker et al., 1995) expressed in migrating lateral line primordia, zebrafish kit is expressed in differentiated sensory cells of lateral line neuromasts. Staining with DASPEI (Parichy, 1996a) does not reveal differences in midbody lateral line neuromast number between spab5 and wild type (F1,15=0.77, P=0.4). Scale bars: (A) 10 μm; (B) 5 μm; (C, D) 30 μm; (E) 500 μm; (F) 40 μm; (G, H) 500 μm; (I) 250 μm; (J) 30 μm; (K) 10 μm. EXPRESSION / LABELING:
|
|
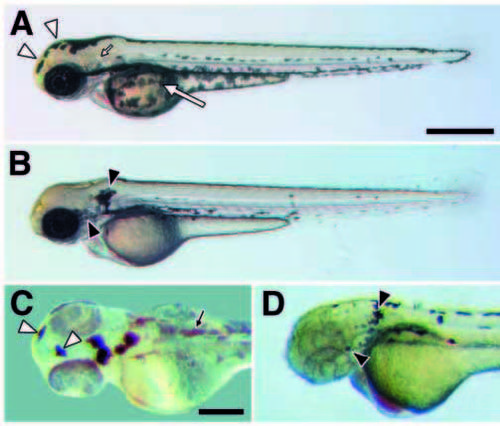
Cell autonomy of kit in promoting melanocyte migration. (A) In wild type (60 hours), melanocytes occur in stripes dorsally at the apex of the myotomes, laterally near the horizontal myoseptum, ventrally at the ventral margin of the myotomes, and covering the yolk mass. (B) In spab5 mutants (60 hours), however, a greater proportion of differentiated melanocytes is found dorsally as compared to wild type (see text); e.g., melanocytes covering the yolk in wild-type embryos (large arrow in A) are absent in sparse (B). Different melanocyte distributions also are evident on the head (Table 1). Melanocytes are abundant over the anterior head of wildtype embryos (12.9% of total; open arrowheads in A) but are virtually absent in spab5 mutant embryos (0.6%); in contrast, melanocytes are abundant lateral to the otocyst (small arrow in A) in spab5 mutant embryos (11.9%; closed arrowheads in B), but are essentially absent in wild-type embryos (0.3%). (C,D) Melanocyte distributions in wild-type–sparse chimeras reveal a cell autonomous role for kit in melanocyte migration. (C) Donor-derived wild-type melanocytes in sparse (spab5, golb1) hosts are abundant over the anterior head (12.8% of total transplanted melanocytes; open arrowheads) but not behind the ear (0.5%), as in wild-type embryos (n=20 chimeras, 415 melanocytes). Small arrow indicates lightly pigmented spab5, golb1 host melanocytes. (D) spab5 mutant melanocytes in wild-type (spa+, albb4) hosts are absent from the anterior head (0%) and accumulate near the otocysts (20.7%; closed arrowhead), as in sparse (n=11 chimeras, 92 cells). Scale bars: (A,B) 500 μm; (C,D) 250 μm. PHENOTYPE:
|
|
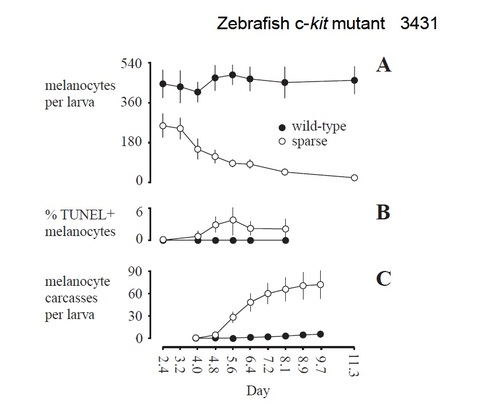
Changes in melanocyte numbers (A), frequencies of apoptotic melanocytes (B) and collection of melanocyte carcasses on nylon membranes (C). (A) In wild type, melanocyte numbers do not differ among days during the first 11 days of development (F7,35=1.20; P=0.3). In spab5 mutants, fewer melanocytes than wild type are present at 2.4 days (60 hours; F1,10=30.87; P<0.001) and these cells are lost over the next several days (F7,33=45.8; P<0.0001). On each day, dorsal stripe melanocytes were counted in 5-7 larvae of each genotype. (B) TUNEL staining is evident in spab5 mutant melanocytes by 4 days and persists at least through 8 days (n=43 larvae, 3903 melanocytes), but is not observed in wild-type melanocytes (n=15 larvae, 2025 melanocytes). TUNEL staining was assessed in dorsal stripe melanocytes in whole-mount, Fast-redstained embryos. (C) The timing of melanocyte carcass recovery on nylon membranes corresponds to the time course of melanocyte loss from spab5 mutant larvae. Error bars in A and B are 95% confidence intervals. Error bars in C are 95% confidence intervals, with intervals summed across days. Counts in A-C were gathered on different days using different groups of larvae and rearing densities. |
|
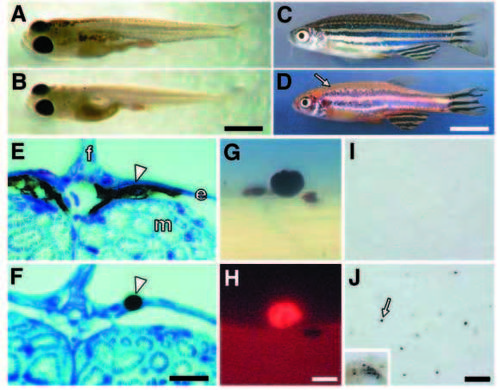
Fate of melanocytes in sparse mutant larvae. (A,B) At 11 days, melanocytes persist in wild type (A), but are virtually absent from spab5 mutants (B). (C,D) sparse mutant larvae recover melanocytes during the larval-to-adult transition. Thus, both wild-type (C) and spab5 (D) adult zebrafish exhibit dark stripes of melanocytes and iridophores, alternating with light stripes of xanthophores and iridophores. Small arrow in D indicates the dorsal region where scale melanocytes are present in wild-type but absent in spab5. (E,F) In 7 day wild-type and sparse mutant larvae, cross sections (3 mm) of plasticembedded specimens reveal a difference in melanocyte localization. In wild type (E), melanocytes (arrowhead) are spread beneath the epidermis; in spab5 mutants (F), however, melanocytes are rounded within the plane of the epidermis (f, fin; e, epidermis; m, myotome). (G,H) sparse mutant melanocytes undergo apoptosis. TUNEL+ melanocytes are rounded and bulging outwards from the epidermis; this melanocyte morphology is not observed in wild-type larvae. Corresponding bright-field (G) and fluorescence (H) images of a spab5 mutant, TUNEL+ melanocyte rounded within the epidermis near the base of the fin fold (5.5 days). (I,J) sparse mutant melanocytes are extruded from the epidermis as revealed by capture on nylon membranes. Membranes beneath free-swimming wild-type larvae is unmarked (I), whereas membranes beneath free-swimming spab5 mutant larvae (J) collect spots of melanin (arrow) of the same size as melanocytes remaining in sparse larvae (inset: same magnification image showing melanocytes on sparse larva dried onto membrane). Scale bars: (A,B) 1 mm; (C,D) 8 mm; (E,F) 20 μm; (G,H) 12 μm; (I,J) 250 μm. |