- Title
-
Antivin, a novel and divergent member of the TGFß superfamily, negatively regulates mesoderm induction
- Authors
- Thisse, C. and Thisse, B.
- Source
- Full text @ Development
|
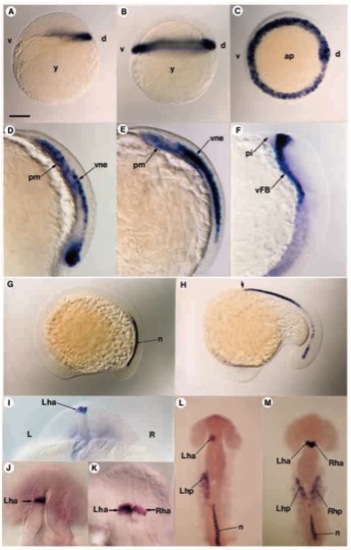
Distribution of antivin transcripts during embryogenesis. (A) atv transcripts are first detected at the sphere stage at the dorsal margin of the embryo. (B,C) 40% epiboly. In lateral view in B and polar view in C, antivin RNA is localized all around the margin. (D) At gastrulation, atv is expressed in the prechordal mesendoderm (pm) and in the overlying ventral neurectoderm (vne). (E) As the mesoderm expressing atv migrates anteriorly, labelling increases in the neurectodermal layer. (F) At the onset of somitogenesis, atv expression is located in the ventral forebrain (vFB) territory. (G) At the 10-somite stage, atv starts to be expressed in the caudal part of the notochord (n). (H) By the 16-somite stage, anterior notochord cells (arrow) express atv, while transcripts disappear posteriorly. (I) At the 20-somite stage, optical cross-section of the diencephalon showing atv expression in the left habenula nucleus (Lha). (J) In 92.8% embryos, atv is expressed in the left part of the habenula. (K) In 4.5% embryos, both left and right sides of the habenula are labelled. (L) When located in the left part of the habenula, atv is also expressed in the left heart primordium (Lhp). (M) When located on both sides of the habenula, atv is also seen in both left and right heart primordia. ap, animal pole; d, dorsal; L, left; pi, pillow; R, right; Rha, right habenula; Rhp, right heart primordium; v, ventral; y, yolk. Scale bar, 150 μm (A,B,D,E,G,H,J,K), 300μm (C,F,I,L), 75μm (M-Q). EXPRESSION / LABELING:
|
|
Morphological defects induced by Antivin overexpression. (A-C,M) Wild-type control (WT). (D-F) Weak phenotypes; (G-I) intermediate phenotypes; (J-L) strong phenotypes; (NQ) range of head defects. (A,D,G,J) Gastrulation stage; (B,E,H,K) somitogenesis; (C,F,I,L) 36 hours of development. (D) Embryos injected with a low dose of antivin RNA (1 pg) are characterized by a shortening and anterior thickening of the embryonic axis. (E) This thickening is located in the prospective head during somitogenesis. (F) The resulting embryos display a lack of cephalic mesoderm (star) and a range of cyclopia phenotypes as detailed in NQ. (G,H) For intermediate doses of RNA (2 pg), the embryos display a thicker and shorter embryonic axis. (I) The derived embryos lack the ventral part of the forebrain, have an unique cyclopic eye (e) and the head is devoid of all mesodermal structures (star). In the absence of notochord, somites (s) fuse under the neural tube. (J) For embryos injected with higher doses of RNA (5 pg), cells accumulate at the margin. (K) The yolk plug closure is located behind the cephalic region. (L) Embryos are defective in all ventral part of the CNS and all mesodermal derivatives of the head and trunk (star). (M-Q) Details of the deletion in the forebrain. The optical vesicles appear closer (N), fused (O-P) or a unique vesicle is formed (Q). Small arrows show the anterior tip of the embryonic axis. Long arrows, position of the yolk plug closure; arrowheads, position of the gastrula margin; n, notochord; ot, otic vesicule. Scale bar: 150 μm (A-C,G,H,L,M); 75 μm (D-F,I-K). |
|
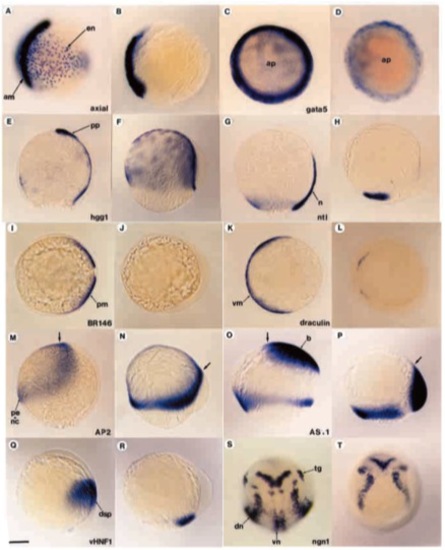
Antivin inhibits mesoderm formation at gastrulation. Embryos injected with antivin RNA were analyzed by in situ hybridization with different molecular probes. For each probe, the wild-type sibling and the corresponding injected embryo are shown next to each other (left and right, respectively). (A,B) Labelling with axial and (C,D) labelling with gata5 for low doses of antivin RNA (1 pg or 0.5 pg, respectively) showing the deletion (A,B) or strong reduction (C,D) of endoderm (en) during gastrulation. (E,F) Labelling with hgg1, a marker of anterior prechordal plate (pp) and yolk syncytial layer. (F) Overstaining with hgg1 reveals the absence of prechordal plate in atv-injected embryos. (G,H) Absence of axial mesoderm is revealed using ntl, which labels the notochord (n) as well as the margin. (I,J) Absence of paraxial mesoderm (pm) is detected probing with BR146. (K,L) draculin, a marker of ventral mesoderm (vm) reveals that atv-injected embryos lack this territory. (M,N) Expression of AP2, specific to presumptive epidermis (pe) and neural crest (nc), is enlarged and shifted dorsovegetally (arrow). (O,P) The presumptive brain (b), labelled with AS11, is located at the margin rather than at the animal pole. (Q,R) Expression of vHNF1, which marks the presumptive dorsal spinal cord (dsp), is shifted to the lateral margin. (S,T) Expression of neurogenin1 (ngn1) reveals the presence of proneural clusters except in the ventral midline. Arrows show the anterior tip of the embryonic axis. dn, dorsal proneural clusters; tg, trigeminal ganglion; vn, ventral proneural clusters. Scale bar: 150 μm. |
|
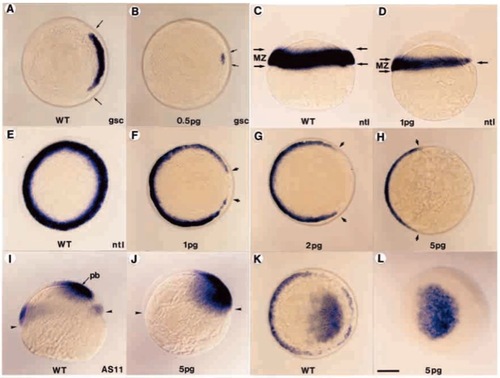
Antivin inhibits mesoderm induction at blastula stage. Wild-type control embryos (A,C,E,I,K) and embryos injected with 0.5 pg (B), 1 pg (D,F), 2 pg (G), 5 pg (H,J,L) antivin RNA were analyzed by in situ hybridization with a marker of prechordal mesoderm, gsc (A,B), a panmesodermal marker, ntl (C-H) or the AS11 marker which labels the margin and the presumptive brain territory (I-L). (B) Injection of antivin RNA inhibits gsc expression (delimited by arrows). (D) Lateral view of an embryo depicting the loss of ntl expression in the marginal zone (MZ, delimited by arrows) along the animal to vegetal pole axis. (F-H) Increasing doses of antivin RNA lead to a progressive deletion (arrows) of the presumptive mesoderm. (H) Detectable expression of ntl is located in cells fated to form the tail bud. (J-L) Labelling with AS11 confirms the complete loss of the marginal zone. Expression of AS11 in presumptive brain (pb) extends down to the margin (arrowheads). Embryos are shown in polar views, except for C,D,I,J, which are lateral views. Scale bar: 150 μm. |
|
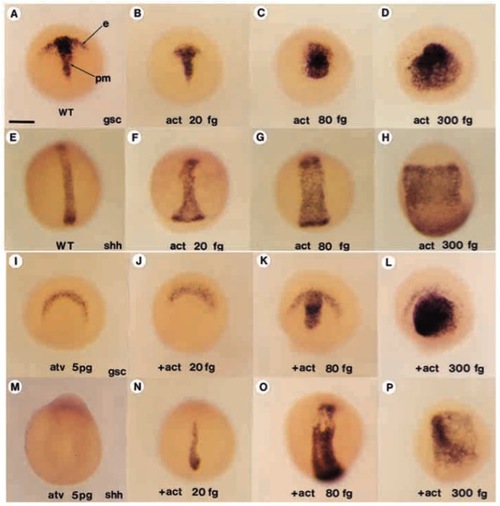
Activin βB rescues the Antivin-mediated deletion of mesoderm. (A-D) Expression of gsc, an anteroaxial mesodermal marker in a wild-type control embryo in A and in embryos injected with increasing doses of activin bB RNA, 20 fg (B), 80 fg (C) or 300 fg (D). (E-H) Expression of shh, a marker of posterior-axial mesoderm, in a wild-type control (E) and in embryos (F-H) injected with the same increasing doses of activin βB. (I) Expression of gsc at the end of gastrulation in an atvinjected embryo. Ectodermal expression of gsc is present while prechordal plate mesodermal expression is absent. (J-L) Coinjection of activin βB has no effect at 20 fg while, at higher doses, the prechordal plate expression of gsc is rescued or even exaggerately induced. (M) atv-injected embryos do not express shh. (N-P) Coinjection of increasing doses of activin βB progressively rescues or even strongly induced shh expression. e, ectodermal expression of gsc; pm, prechordal mesoderm. Scale bar: 200 μm. |
|
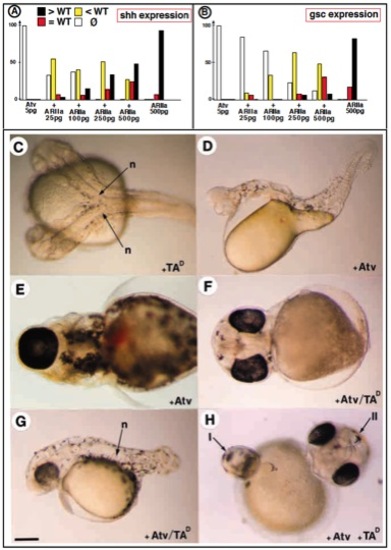
Rescue of the Antivin-mediated loss of mesoderm by Activin Receptor type IIA and Taram AD. (A,B) Rescue of the Atv inhibitory effect by coinjection of increasing doses of Activin receptor type IIa. Bars indicate the percentage of embryos displaying an overinduced expression (black), a wild-type expression (red), a reduced expression (yellow), or a complete loss of expression (white) of shh, (A), or of gsc (B). (C) Embryo injected with 500 pg of TARAM-AD RNA in one blastomere at the 16-cell stage develops a complete secondary axis including a fully differentiated notochord (n). (D) Embryo injected with 10 pg of antivin RNA showing a lack of all mesendodermal derivatives in head and trunk and an absence of axial mesoderm in tail. (E) Focus on the cyclopia phenotype of an embryo injected with 10 pg of Atv RNA (ventral view). (F) Coinjection of 500 pg of TARAM-AD RNA rescues Atv-mediated cyclopia phenotype and (G) a differentiated notochord. (H) Front view of an embryo injected with Atv (10 pg) at the 2-cell-stage followed by injection of TARAM-AD RNA (500 pg) in a marginal blastomere at the 16-cell-stage. Two axes are formed, the primary axis (I) is devoid of mesendoderm and displays a cyclopia phenotype as the result of Atv effect while the secondary axis (II) induced by TARAM-AD comprises axial mesendoderm and two separated eyes. Scale bar: 150 μm in C,D,G; 100 μm in E,F,H. |