- Title
-
Temporal separation in the specification of primary and secondary motoneurons in zebrafish
- Authors
- Beattie, C.E., Hatta, K., Halpern, M.E., Liu, H., Eisen, J.S., and Kimmel, C.B.
- Source
- Full text @ Dev. Biol.
|
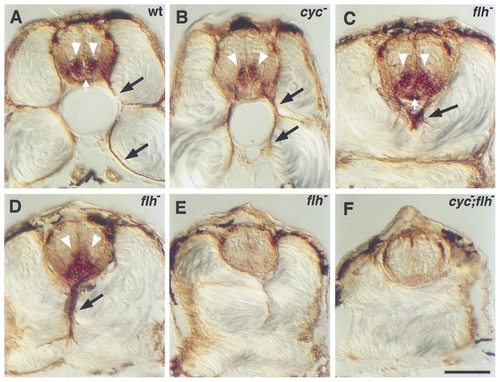
Secondary motoneurons in mutant embryos. Antibody labeling (zn-5) of the Dm-grasp protein was used to characterize secondary motoneurons and floor plate in transverse sections through the trunk of wild-type and mutant embryos at 48 hr. Wild-type embryos (A) have two columns of secondary motoneuronal cell bodies in the ventral spinal cord (white arrowheads) and axons extending ventrally out of the spinal cord (black arrows). The floor plate, a triangular group of cells ventral to the secondary motoneuronal cell bodies, is also labeled (white arrow). In cyc mutants (B), the secondary motoneuronal cell bodies are often fused at the midline, but axons still extend out of the spinal cord. Floor plate is absent. In flh mutants (C, D, E), three patterns were observed. In some cases (C), the secondary motoneuronal cell bodies look normal and are separated by floor plate (white arrow). In other cases (D), bilateral clusters of cell bodies are fused across the midline and floor plate is visible. In both of these cases, axons extend out of the spinal cord, but their trajectories are aberrant (black arrows in C and D). In the third case (E), there are no secondary motoneuronal cell bodies, no axons, and no floor plate. In cyc;flh mutants (F), secondary motoneuronal cell bodies and axons as well as floor plate are absent. Scale bar, 50 μm. |
|
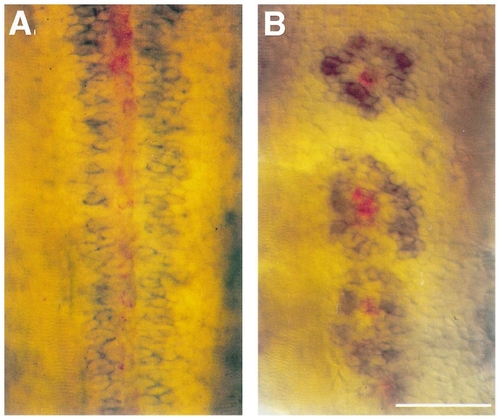
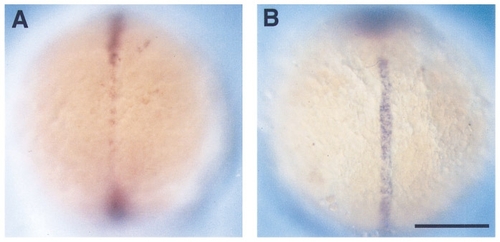
Secondary motoneurons form rings around floor plate cells in flh mutant embryos. Double RNA in situ hybridizations at 36 hr with antisense probes against dm-grasp labeling secondary motoneurons (blue) and col2a1 labeling floor plate (red). Dorsal view of wildtype embryos reveals that secondary motoneurons form bilateral rows flanking the continuous floor plate (A). In flh mutants, secondary motoneurons form rings around the floor plate islands (B; n = 14 embryos, 142 floor plate islands). Note that while the antibody to Dm-grasp (Zn-5) labels both secondary motoneurons and floor plate (Fig. 1), the antisense probe to dm-grasp RNA has not been reported to label floor plate (Kanki et al., 1994). Scale bar, 50 μm. |
|
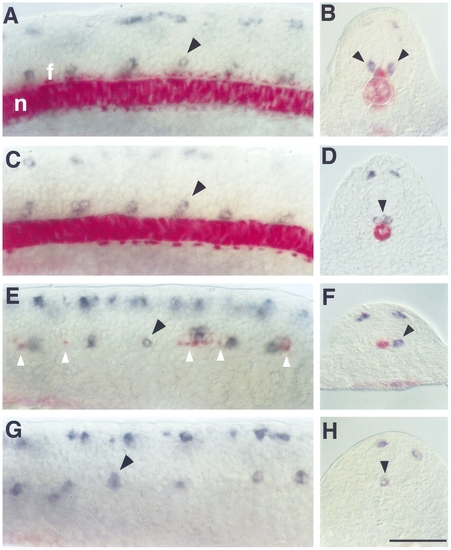
Primary motoneurons develop in the absence of floor plate and notochord. Double RNA in situ hybridizations at 18 hr with antisense probes against islet2, a gene expressed in two of the primary motoneurons, CaP and VaP, in blue and col2a1, a gene expressed in floor plate and notochord, in red. Lateral views (A, C, E, G; dorsal to the top and anterior to the left), transverse sections (B, D, F, H). In wild types the notochord (n) and floor plate (f) are present and continuous as revealed by col2a1 expression (A, B). One or two islet2- expressing primary motoneurons are present in the ventral region of each spinal cord segment (black arrowhead). islet2-expressing cells in the dorsal spinal cord are Rohon–Beard sensory neurons. In cyc mutants (C, D) notochord is present but floor plate is absent. The pattern of islet2 expression in the ventral spinal cord is comparable to that seen in wild types, except that the cell bodies are often collapsed at the midline (black arrowhead in D). In flh mutants (E, F) notochord is missing and floor plate is present as discontinuous islands of cells (white arrowheads). One or two islet2-expressing primary motoneurons are present in each segment, most are associated with floor plate, but some are present in segments containing no floor plate (black arrowhead in E). In cyc;flh mutants (G, H) both notochord and floor plate are absent. There are, however, islet2-expressing primary motoneurons present in the ventral spinal cord (black arrowheads). Scale bar, 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
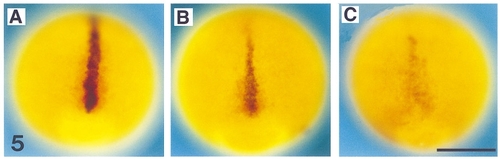
shh expression in flh and cyc;flh mutants during late gastrulation. Dorsal views (anterior to the top) of whole-mount RNA in situ hybridization at 90% epiboly (9 hr) with antisense probes against shh in blue and myoD in red. At 90% epiboly shh is present in the midline of wild-type embryos (A). In flh mutants, shh is still present at the midline, but the expression levels are decreased compared to wild types (B). In cyc;flh mutants, the shh expression levels are dramatically decreased, but consistently present (C). myoD expression is in regions that will become paraxial mesoderm indicating that the shh expression corresponds to the trunk region of the embryo. Scale bar, 250 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
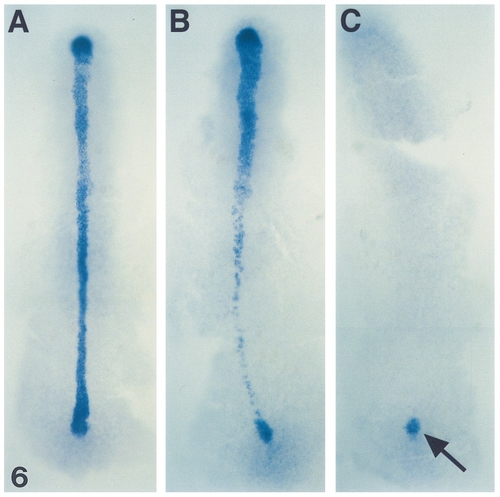
shh expression in flh and cyc;flh mutants during early segmentation. Dorsal views (anterior to the top) of whole-mount RNA in situ hybridization at the 3-somite stage (11 hr) with an antisense probe against shh in blue. shh expression in wild types is present in the forming notochord and floor plate (A). In flh mutants, shh expression is patchy in what will be the trunk region, reflecting the missing notochord and the discontinuous floor plate (B). In cyc;flh mutants, shh expression is completely undetected in the trunk (C) with the only detectable expression in the tail bud (arrow). EXPRESSION / LABELING:
PHENOTYPE:
|
|
ehh and twhh are present in single mutants but absent from the double mutant. Dorsal views (anterior to the top) of wholemount RNA in situ hybridizations at the 3-somite stage (11 hr). (A) flh mutant embryo hybridized with an antisense probe against twhh. (B) cyc mutant embryo hybridized with an antisense probe against ehh. cyc-;flh- double mutants’ lack both twhh and ehh at this stage (Table 1). Scale bar, 200 μm. EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 187(2), Beattie, C.E., Hatta, K., Halpern, M.E., Liu, H., Eisen, J.S., and Kimmel, C.B., Temporal separation in the specification of primary and secondary motoneurons in zebrafish, 171-182, Copyright (1997) with permission from Elsevier. Full text @ Dev. Biol.